Table of Contents
Physical and Chemical Changes- Worksheets
These worksheets are based on the chapter “Physical and Chemical Changes.” In this chapter, we explore how substances undergo changes in their physical and chemical properties. The worksheets are available in both solved and unsolved versions to assist in learning and understanding these concepts.
Key topics covered in the chapter and addressed in the worksheets include-
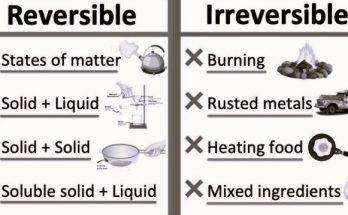
- Physical Changes- Changes where a substance’s physical properties, like shape or state, change without forming a new substance.
- Chemical Changes- Changes that result in new substances being formed, such as rusting, burning, or cooking.
- Experiments and Examples- Practical activities and examples to differentiate between physical and chemical changes.
The solved worksheets provide solutions and explanations, helping students understand the concepts. The unsolved worksheets offer practice opportunities to apply and assess their knowledge.
Also Check -Rapid Revision – Class 7 Science- Chapter 6- Physical and Chemical Changes
Worksheet 1 – Physical and Chemical Changes – Student Worksheet (Unsolved)
State differences between physical and chemical changes.
Explain how the souring of milk is a chemical change.
What are the characteristics of physical changes? Give two examples.
Why is rusting of iron considered a chemical change? What are the substances involved in this process?
Is cutting of paper a physical or chemical change? Justify your answer.
Mark ‘T’ if the statement is true and ‘F’ if it is false-
- (i) Boiling of water is a chemical change. (T/F)
- (ii) Burning of wood is a reversible change. (T/F)
- (iii) Rusting of iron produces a new substance. (T/F)
- (iv) Stretching a rubber band involves change in its chemical properties. (T/F)
- (v) Melting of ice cream is a chemical change. (T/F)
Describe the process of neutralisation with the help of an example involving acids and bases.
Explain why-
(a) Galvanization is used to prevent rusting of iron.
(b) Photosynthesis is a chemical change.
(c) Spoilage of food is considered a chemical change.
How does the process of digestion represent a chemical change?
What is crystallisation, and how is it different from a chemical change? Provide an example.
Consider the following statements about physical and chemical changes-
- (a) Physical changes usually involve energy changes.
- (b) Chemical changes always result in the formation of new substances.
- (c) All changes in state of matter are physical changes.
- (d) Dissolving sugar in water is a chemical change.
Which of these statements are correct? (i) All four (ii) b and c (iii) a, b and c (iv) only b
Discuss the environmental implications of chemical changes like acid rain and rusting.
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Worksheet 1 – Physical and Chemical Changes – Solved Worksheet
State differences between physical and chemical changes.
- Physical changes are usually reversible and don’t result in the formation of new substances, such as melting of ice or tearing of paper. Chemical changes, on the other hand, result in new substances being formed and are often irreversible, like the rusting of iron or the burning of wood.
Explain how the souring of milk is a chemical change.
- Souring of milk is a chemical change because it involves the transformation of lactose (milk sugar) into lactic acid due to bacterial action. This process results in a new substance (lactic acid) and is irreversible, characteristic of a chemical change.
What are the characteristics of physical changes? Give two examples.
- Characteristics of physical changes include no new substance formation, often being reversible, and usually only involving a change in physical properties like shape or state. Examples include the dissolving of salt in water and the shredding of paper.
Why is rusting of iron considered a chemical change? What are the substances involved in this process?
- Rusting of iron is a chemical change because it results in a new substance- iron oxide. It involves iron and oxygen reacting in the presence of water or moisture, and the process is irreversible.
Is cutting of paper a physical or chemical change? Justify your answer.
- Cutting paper is a physical change. It alters the size and shape of the paper but doesn’t change its chemical composition. The paper remains chemically the same before and after cutting.
Mark ‘T’ if the statement is true and ‘F’ if it is false-
- (i) Boiling of water is a chemical change. (F)
- (ii) Burning of wood is a reversible change. (F)
- (iii) Rusting of iron produces a new substance. (T)
- (iv) Stretching a rubber band involves change in its chemical properties. (F)
- (v) Melting of ice cream is a chemical change. (F)
Describe the process of neutralisation with the help of an example involving acids and bases.
- Neutralisation is the reaction between an acid and a base to form salt and water. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), they neutralise each other, forming sodium chloride (NaCl) and water (H2O).
Also Check – What is Neutralisation? – The Basics Explained
Explain why-
- (a) Galvanization is used to prevent rusting of iron because it involves coating iron with a layer of zinc, which is more reactive to oxygen. This prevents iron from oxidising.
- (b) Photosynthesis is a chemical change as it transforms carbon dioxide and water into glucose and oxygen using sunlight, resulting in new substances.
- (c) Spoilage of food is considered a chemical change because it involves the breakdown of food substances into new compounds by microorganisms or enzymes.
How does the process of digestion represent a chemical change?
- Digestion is a chemical change because it involves the breakdown of complex food substances into simpler substances through chemical reactions in the body, such as the conversion of starch into glucose.
What is crystallisation, and how is it different from a chemical change? Provide an example.
- Crystallisation is a physical change where a solid forms from a solution or from the solid-liquid state, maintaining the chemical composition of the substance. An example is the formation of salt crystals from evaporating seawater.
Consider the following statements about physical and chemical changes-
- Correct option- (iii) a, b, and c
Discuss the environmental implications of chemical changes like acid rain and rusting.
- Acid rain, resulting from chemical reactions in the atmosphere involving pollutants, can damage ecosystems, corrode buildings, and harm aquatic life. Rusting weakens metal structures, leading to safety hazards and economic losses.
Also Check – 50 Examples of Physical Changes
Worksheet 2 – Physical and Chemical Changes – Student Worksheet (Unsolved)
Explain how cooking food is a chemical change.
Describe the role of heat in both physical and chemical changes, providing two distinct examples.
What is sublimation? Give two examples of substances that undergo sublimation.
Why is the ripening of fruit considered a chemical change?
Is dissolving sugar in water a physical or chemical change? Justify your answer.
Mark ‘T’ if the statement is true and ‘F’ if it is false-
- (i) Condensation of steam is a chemical change. (T/F)
- (ii) Burning of candles results in both physical and chemical changes. (T/F)
- (iii) Fermentation of grapes to wine is a reversible change. (T/F)
- (iv) Formation of manure from leaves is a physical change. (T/F)
- (v) Crystallisation of salt from seawater is a chemical change. (T/F)
Discuss how rusting can be prevented, explaining the chemical process involved in rusting.
Explain why-
- (a) Paper burning is a chemical change.
- (b) Freezing of water is a physical change.
- (c) Digestion of food involves both physical and chemical changes.
What is the significance of chemical changes in the environment? Give two examples.
How do physical changes differ from chemical changes in terms of energy absorption or release?
Consider the following statements about changes in matter-
- (a) All chemical changes are accompanied by energy changes.
- (b) Physical changes always result in the formation of new substances.
- (c) Chemical changes are generally irreversible.
- (d) Dissolving of salt in water is a chemical change.
Which of these statements are correct? (i) All four (ii) a and c (iii) b and d (iv) only c
Explain the concept of reversible and irreversible changes with suitable examples.
Also Check – 20 Plus List of Chemical Changes Examples
Worksheet 2 – Physical and Chemical Changes – Solved Worksheet
Explain how cooking food is a chemical change.
- Cooking food is a chemical change as it involves changes at the molecular level, where new substances are formed. For example, when meat is cooked, it undergoes chemical reactions that change its structure, flavour, and colour. These changes are irreversible, indicating a chemical change.
Describe the role of heat in both physical and chemical changes, providing two distinct examples.
- In physical changes, heat can change the state of a substance without altering its chemical composition, like melting ice into water. In chemical changes, heat can initiate or accelerate chemical reactions, such as when heat causes sugar to caramelise or metal to rust.
What is sublimation? Give two examples of substances that undergo sublimation.
- Sublimation is the process where a substance transitions directly from a solid to a gas without passing through the liquid state. Examples include dry ice (solid carbon dioxide) and iodine.
Why is the ripening of fruit considered a chemical change?
- Ripening of fruit is a chemical change because it involves chemical reactions that alter the fruit’s composition, such as converting starches to sugars, which changes the taste, texture, and colour of the fruit.
Is dissolving sugar in water a physical or chemical change? Justify your answer.
- Dissolving sugar in water is a physical change. It’s a process of mixing where sugar molecules are spread out in the water but their chemical structure doesn’t change. The process is generally reversible through evaporation.
Mark ‘T’ if the statement is true and ‘F’ if it is false-
- (i) Condensation of steam is a chemical change. (F)
- (ii) Burning of candles results in both physical and chemical changes. (T)
- (iii) Fermentation of grapes to wine is a reversible change. (F)
- (iv) Formation of manure from leaves is a physical change. (F)
- (v) Crystallisation of salt from seawater is a chemical change. (F)
Discuss how rusting can be prevented, explaining the chemical process involved in rusting.
- Rusting can be prevented through methods like galvanization, painting, or using rust-resistant alloys. Rusting is a chemical process where iron reacts with oxygen and water to form iron oxide (rust), which is destructive and irreversible.
Explain why-
- (a) Paper burning is a chemical change because it results in the formation of new substances like ash, water vapour, and carbon dioxide.
- (b) Freezing of water is a physical change as it only changes the state of water from liquid to solid without altering its chemical composition.
- (c) Digestion of food involves both physical changes (like grinding in the mouth) and chemical changes (like enzymatic breakdown in the stomach and intestines).
What is the significance of chemical changes in the environment? Give two examples.
- Chemical changes are significant in the environment as they can lead to both beneficial and harmful effects. Examples include photosynthesis (beneficial) and pollution from industrial emissions leading to acid rain (harmful).
How do physical changes differ from chemical changes in terms of energy absorption or release?
- In physical changes, energy changes are usually less dramatic and often reversible, like melting or freezing. In chemical changes, energy is either absorbed or released in more significant amounts, and the change is often irreversible, like in combustion.
Consider the following statements about changes in matter-
- The correct option- (ii) a and c
Explain the concept of reversible and irreversible changes with suitable examples.
- Reversible changes are those where the original form can be restored, like melting and freezing of water. Irreversible changes are where the original form cannot be restored, like the burning of paper.
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Worksheet 3 – Physical and Chemical Changes – Student Worksheet (Unsolved)
Define a physical change and a chemical change. Give two examples of each from your daily life.
Explain why rusting of iron is a chemical change. What are the reactants and the product of this change?
Describe the process and result of burning a candle. Is it a physical or chemical change? Why?
What happens during the ripening of fruits? Classify this process as a physical or chemical change and justify your answer.
Is dissolving salt in water a physical or chemical change? Explain your reasoning.
Mark ‘T’ if the statement is true and ‘F’ if it is false-
- (i) Crystallisation is a chemical change. (T/F)
- (ii) Boiling of water is a chemical change. (T/F)
- (iii) Digestion of food is a physical change. (T/F)
- (iv) Rusting of iron can be reversed easily. (T/F)
- (v) Photosynthesis is a physical change. (T/F)
Discuss the importance of chemical changes in industrial processes, providing two examples.
Explain why-
- (a) Cutting a piece of paper is considered a physical change.
- (b) Cooking an egg results in a chemical change.
- (c) Freezing of water is a physical change.
How does the process of condensation demonstrate a physical change? Provide an example.
What are the environmental implications of rusting?
Consider the following statements and indicate whether they refer to a physical or chemical change-
- (a) Melting butter in a pan.
- (b) Souring of milk.
- (c) Dissolving sugar in tea.
- (d) Formation of wine from grapes.
Explain with an example how a chemical change can impact our environment.
Worksheet 3 – Physical and Chemical Changes – Solved Worksheet
Define a physical change and a chemical change. Give two examples of each from your daily life.
- Physical change- A change where the substance undergoes a change in its physical properties but not its chemical composition. Examples- Melting of ice cream, folding a paper.
- Chemical change- A change where the substance undergoes a change in its chemical composition, resulting in the formation of new substances. Examples- Rusting of iron, baking a cake.
Explain why rusting of iron is a chemical change. What are the reactants and the product of this change?
- Rusting of iron is a chemical change as it results in a new substance, iron oxide, being formed from iron and oxygen in the presence of water or moisture. The reactants are iron (Fe) and oxygen (O2), and the product is iron oxide (Fe2O3).
Describe the process and result of burning a candle. Is it a physical or chemical change? Why?
- Burning a candle involves both physical and chemical changes. The physical change is the melting of the wax. The chemical change is the burning of the wax (hydrocarbons) in the presence of oxygen, producing carbon dioxide, water vapour, heat, and light. It’s a chemical change because new substances are formed.
What happens during the ripening of fruits? Classify this process as a physical or chemical change and justify your answer.
- During the ripening of fruits, complex chemicals like starch are converted into simpler forms like sugar. This process is a chemical change as new substances (sugars) are formed, and the change is irreversible.
Is dissolving salt in water a physical or chemical change? Explain your reasoning.
- Dissolving salt in water is a physical change. The salt (sodium chloride) dissociates into its ions but doesn’t change its chemical structure. The process is generally reversible by evaporating the water.
Mark ‘T’ if the statement is true and ‘F’ if it is false-
- (i) Crystallisation is a chemical change. (F)
- (ii) Boiling of water is a chemical change. (F)
- (iii) Digestion of food is a physical change. (F) – It’s both physical and chemical.
- (iv) Rusting of iron can be reversed easily. (F)
- (v) Photosynthesis is a physical change. (F)
Discuss the importance of chemical changes in industrial processes, providing two examples.
- Chemical changes are crucial in industrial processes as they lead to the production of new materials. Examples- Manufacture of steel from iron ore (reduction process), production of plastic from petrochemicals.
Explain why-
- (a) Cutting a piece of paper is considered a physical change because it alters the paper’s shape and size but not its chemical composition.
- (b) Cooking an egg results in a chemical change as it changes the protein structure in the egg, forming a new substance.
- (c) Freezing of water is a physical change as it only changes the state of water from liquid to solid without altering its chemical composition.
How does the process of condensation demonstrate a physical change? Provide an example.
- Condensation, like the formation of water droplets from water vapour, is a physical change. It’s a change of state from gas to liquid without altering the chemical composition of water.
What are the environmental implications of rusting?
- Rusting can weaken metal structures like bridges and vehicles, leading to safety hazards and economic loss. It also indicates the consumption of metals in the environment.
Consider the following statements and indicate whether they refer to a physical or chemical change-
- (a) Melting butter in a pan. (Physical Change)
- (b) Souring of milk. (Chemical Change)
- (c) Dissolving sugar in tea. (Physical Change)
- (d) Formation of wine from grapes. (Chemical Change)
Explain with an example how a chemical change can impact our environment.
- An example is the combustion of fossil fuels, which leads to the release of greenhouse gases like carbon dioxide, contributing to global warming and air pollution.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Worksheet 4 – Physical and Chemical Changes – Student Worksheet (Unsolved)
Differentiate between reversible and irreversible changes with two examples for each.
Why is burning paper considered a chemical change? What new substances are formed?
Describe the change that occurs when vinegar reacts with baking soda. Is it a physical or chemical change?
What is sublimation? List two substances that undergo this process.
Explain the concept of conservation of mass in a chemical change with an example.
True or False-
- (i) Dissolving carbon dioxide in water is a chemical change. (T/F)
- (ii) Formation of curd from milk can be reversed by heating. (T/F)
- (iii) Precipitation is a result of a chemical change in the atmosphere. (T/F)
- (iv) Breaking a glass bottle results in a new substance being formed. (T/F)
- (v) Fermentation to produce alcohol is a physical change. (T/F)
How does the process of electrolysis illustrate a chemical change? Give an example.
Explain why-
- (a) Bending of a metal rod is a physical change.
- (b) Formation of yoghurt from milk is a chemical change.
- (c) Melting of wax is a physical change, while burning it is a chemical change.
Discuss the role of temperature in affecting physical and chemical changes. Provide two examples for each.
What are the characteristics of a chemical reaction that distinguish it from a physical change?
Explain how cooking transforms the physical and chemical properties of food.
Describe how a physical change can lead to a chemical change, using the process of cooking as an example.
Also Check – Crystallisation – Class 7
Worksheet 4 – Physical and Chemical Changes – Solved Worksheet
Differentiate between reversible and irreversible changes with two examples for each.
- Reversible changes- These are changes that can be undone. Examples- Melting of ice (can be frozen back to ice), stretching a rubber band (returns to original shape when released).
- Irreversible changes- Changes that cannot be undone. Examples- Burning of paper (cannot be reversed), rusting of iron (cannot be reverted to original iron).
Why is burning paper considered a chemical change? What new substances are formed?
- Burning of paper is a chemical change because it results in the formation of new substances that cannot be turned back into paper. When paper burns, it reacts with oxygen to form carbon dioxide, water vapour, ash, and releases energy in the form of light and heat.
Describe the change that occurs when vinegar reacts with baking soda. Is it a physical or chemical change?
- When vinegar (acetic acid) reacts with baking soda (sodium bicarbonate), it’s a chemical change. This reaction produces carbon dioxide gas, water, and sodium acetate. The formation of gas bubbles is evidence of this chemical reaction.
What is sublimation? List two substances that undergo this process.
- Sublimation is the process where a substance changes directly from a solid to a gas without passing through the liquid phase. Examples- Dry ice (solid carbon dioxide) and iodine.
Explain the concept of conservation of mass in a chemical change with an example.
- The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. For example, when hydrogen gas reacts with oxygen to form water, the total mass of water produced is equal to the sum of the masses of hydrogen and oxygen that reacted.
True or False-
- (i) Dissolving carbon dioxide in water is a chemical change. (T)
- (ii) Formation of curd from milk can be reversed by heating. (F)
- (iii) Precipitation is a result of a chemical change in the atmosphere. (F)
- (iv) Breaking a glass bottle results in a new substance being formed. (F)
- (v) Fermentation to produce alcohol is a physical change. (F)
How does the process of electrolysis illustrate a chemical change? Give an example.
- Electrolysis is a chemical change where an electric current causes a chemical reaction. For example, in the electrolysis of water, water is decomposed into hydrogen and oxygen gases.
Explain why-
- (a) Bending a metal rod is a physical change because the rod’s composition remains the same; only its shape changes.
- (b) Formation of yoghurt from milk is a chemical change as it involves the conversion of lactose to lactic acid by bacteria, resulting in a new substance.
- (c) Melting of wax is a physical change (state change), but burning it is a chemical change (new substances are formed).
Discuss the role of temperature in affecting physical and chemical changes. Provide two examples for each.
- Physical changes- Temperature can change the state of matter (melting ice, boiling water).
- Chemical changes- Temperature can affect reaction rates (increasing temperature speeds up chemical reactions, like cooking food or rusting iron).
What are the characteristics of a chemical reaction that distinguish it from a physical change?
- A chemical reaction involves the formation of new substances, is often irreversible, and may involve energy changes (heat, light).
Explain how cooking transforms the physical and chemical properties of food.
- Cooking alters the texture and taste of food (physical change) and also causes chemical reactions that change the nutritional content and flavour (chemical change).
Describe how a physical change can lead to a chemical change, using the process of cooking as an example.
- In cooking, physical changes like cutting or blending can increase the surface area of food, leading to more effective chemical reactions during cooking, like caramelization or protein denaturation.
Also Check – Difference between Physical and Chemical Change
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)