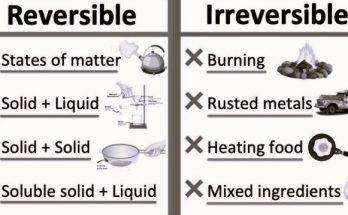
Chapter – 5 -Physical and Chemical Changes- Concepts included in the chapter –
- Physical Changes- These are changes where the substance itself remains the same despite its altered state, shape, or form. Examples include melting ice, tearing paper, and dissolving sugar in water.
- Chemical Changes- These changes result in the formation of new substances. The chapter discusses examples like the rusting of iron, the reaction of vinegar and baking soda, and the burning of magnesium ribbon.
- Rusting of Iron- A detailed examination of how iron corrodes, forming a new substance – rust.
Crystallisation- This section covers how pure crystals are formed from solutions, showcasing the process with substances like copper sulphate.
NCERT Exemplar Class 7 Science Chapter – 5-Physical and Chemical Changes- (Solved with detailed explanation)
MULTIPLE CHOICE QUESTIONS
1. Which of the following is a physical change?
- (a) Rusting of iron
- (b) Combustion of magnesium ribbon
- (c) Burning of a candle
- (d) Melting of wax
Answer- (d) Melting of wax
Explanation- Melting of wax involves a change in the state of matter from solid to liquid without altering its chemical composition. It is a physical change.
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
2. Which of the following is a chemical change?
- (a) Twinkling of stars
- (b) Cooking of vegetables
- (c) Cutting of fruits
- (d) Boiling of water
Answer- (b) Cooking of vegetables
Explanation- Cooking of vegetables involves chemical reactions between the food components and heat, resulting in the formation of new substances with different properties. It is a chemical change.
Also Check – Rapid Revision – Class 7 Science- Chapter 6- Physical and Chemical Changes
3. A chemical change may involve –
- (a) Change in colour only
- (b) Change in temperature only
- (c) Evolution of gas only
- (d) Any or all of the above
Answer- (d) Any or all of the above
Explanation- Chemical changes can involve changes in colour, temperature, evolution of gas, or any combination of these. They are diverse in nature.
4. Which of the following is/are true when milk changes into curd?
- (i) Its state is changed from liquid to semi-solid.
- (ii) It changes colour.
- (iii) It changes taste.
- (iv) The change cannot be reversed.
Answer- (b) (ii) and (iii) are correct
Explanation- When milk changes into curd, it does change in colour and taste, but the change can be reversed by adding an acid or curd culture to the milk.
5. A man painted his main gate made up of iron, to
- (i) Prevent it from rusting.
- (ii) Protect it from the sun.
- (iii) Make it look beautiful.
- (iv) Make it dust-free.
Answer- (a) (i) and (iii)
Explanation- The main purpose of painting an iron gate is to prevent it from rusting (chemical change) and to enhance its appearance.
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
6. Iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
- (a) It is more than 7 metres high.
- (b) It weighs about 6000 kg.
- (c) It was built more than 1600 years ago.
- (d) It has not rusted after such a long period.
Answer- (d) It has not rusted after such a long period.
Explanation- The iron pillar’s long stability is due to its resistance to rusting, making it a remarkable example of iron’s durability.
7. Galvanization is a process used to prevent the rusting of which of the following?
- (a) Iron
- (b) Zinc
- (c) Aluminium
- (d) Copper
Answer- (a) Iron
Explanation- Galvanization is a process used to prevent the rusting of iron or steel objects by applying a protective layer of zinc.
8. Paheli’s mother made a concentrated sugar syrup by dissolving sugar in hot water. On cooling, crystals of sugar got separated. This indicates a –
- (a) Physical change that can be reversed.
- (b) Chemical change that can be reversed.
- (c) Physical change that cannot be reversed.
- (d) Chemical change that cannot be reversed.
Answer- (a) Physical change that can be reversed.
Explanation– The formation of sugar crystals from a concentrated syrup is a physical change that can be reversed by dissolving the crystals in water again.
Also Check – 50 Examples of Physical Changes
9. Which of the following statements is incorrect for a chemical reaction?
- (a) Heat may be given out but never absorbed.
- (b) Sound may be produced.
- (c) A colour change may take place.
- (d) A gas may be evolved.
Answer- (a) Heat may be given out but never absorbed.
Explanation- In a chemical reaction, heat can either be given out (exothermic) or absorbed (endothermic), depending on the reaction. So, the statement is incorrect.
10. Two drops of dilute sulfuric acid were added to 1 g of copper sulphate powder, and then a small amount of hot water was added to dissolve it (step I). On cooling, beautiful blue-coloured crystals got separated (step II). Step I and step II are-
- (a) Physical and chemical changes, respectively.
- (b) Chemical and physical changes, respectively.
- (c) Both physical changes.
- (d) Both chemical changes.
Answer- (b) Chemical and physical changes, respectively.
Explanation- In step I, a chemical change occurs as sulfuric acid reacts with copper sulphate. In step II, the formation of blue crystals is a physical change.
11. State whether the following statements are true or false-
(a) When a candle burns, both physical and chemical changes take place.
Answer- True
Explanation- When a candle burns, it undergoes both physical and chemical changes simultaneously. The physical change involves the melting of the wax, which changes its state from solid to liquid. The chemical change is the combustion of the wax, where it reacts with oxygen in the air to produce heat, light, water vapour, and carbon dioxide gas. So, both types of changes occur during the burning of a candle.
(b) Anaerobic bacteria digest animal waste and produce biogas.
Answer- True
Explanation- This statement is true. Anaerobic bacteria can digest organic matter found in animal waste in the absence of oxygen. During this digestion process, they produce biogas, which is a mixture of methane and carbon dioxide. It is a valuable source of renewable energy.
(c) Ships suffer a lot of damage though they are painted.
Answer- True
Explanation- This statement is true. Ships, especially those in saltwater environments, can still suffer from rust and corrosion even when they are painted. The saltwater and constant exposure to moisture can lead to rusting over time, causing damage to the ship’s structure.
(d) Stretching of a rubber band is not a physical change.
Answer- False
Explanation- This statement is false. Stretching of a rubber band is indeed a physical change. It involves altering the shape and size of the rubber band without changing its chemical composition.
12. Melting of wax is a change where a solid changes to a liquid state. Give one more such change which you observe in your surroundings.
Answer- Freezing of water is another change where a liquid (water) changes to a solid (ice) state. This change is commonly observed when water freezes at low temperatures.
13. What kind of change is shown by the tearing of paper?
Answer- The tearing of paper is a physical change. It involves the alteration of the paper’s shape and size without changing its chemical composition.
14. Match the items of Column I with the items of Column II.
Answer-
| Column I | Column II |
|---|---|
| (a) Large crystals | (iii) Sugar candy (Mishri) – Physical change |
| (b) Turns lime water milky | (iv) Carbon dioxide – Chemical change |
| (c) Depositing a layer of zinc on iron | (vi) Galvanisation – Physical change |
| (d) Souring of milk | (i) Iron oxide – Chemical change |
| (e) Rust | (ii) Depositing a layer of zinc on iron – Physical change |
| (f) Dissolving common salt in water | (v) Turns lime water milky – Chemical change |
Explanation-
(a) Large crystals turning into sugar candy (Mishri) involves a physical change as it changes the form and size of the crystals without altering their chemical composition.
(b) When a substance turns lime water milky, it indicates a chemical change. Carbon dioxide gas reacts with calcium hydroxide in lime water to form calcium carbonate, which makes it milky.
(c) Depositing a layer of zinc on iron through galvanisation is a physical change. It changes the appearance and properties of the iron’s surface but doesn’t alter its chemical composition.
(d) Souring of milk is a chemical change. The bacteria in milk produce lactic acid, changing its taste and composition.
(e) Rusting of iron involves a chemical change. Iron reacts with oxygen and moisture to form iron oxide, which is rust.
(f) Dissolving common salt (sodium chloride) in water is a physical change. It doesn’t change the chemical composition of either the salt or the water; it only involves mixing.
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Question 15-
Fill in the blanks in the following statements using the words given in the box.
Boxed Words- rusted, colourful, substance, chemical, physical, reversible, iron oxide, object
(a) Making sugar solution is a ____________ change.
Answer- Making sugar solution is a physical change.
Explanation- When sugar dissolves in water to form a solution, it is a physical change because it does not involve the formation of new substances. The sugar molecules remain the same; only their physical arrangement in the solution changes.
(b) A physical change is generally ____________.
Answer- A physical change is generally reversible.
Explanation- Physical changes typically do not alter the chemical composition of substances, making them reversible. You can usually return the substance to its original state by reversing the conditions that caused the change.
(c) Grinding of wheat grain changes its size. It is a ____________ change.
Answer- Grinding of wheat grain changes its size. It is a physical change.
Explanation- Grinding wheat grains only changes their physical appearance (size) without altering their chemical composition, so it is considered a physical change.
(d) Iron benches kept in lawns and gardens get ____________. It is a _________ change because a new _________ is formed.
Answer- Iron benches kept in lawns and gardens get rusted. It is a chemical change because a new substance (iron oxide) is formed.
Explanation- Rusting of iron is a chemical change because iron reacts with oxygen and moisture to form iron oxide, which has different properties from pure iron. This change is not reversible without undergoing another chemical reaction.
Also Check – 20 Plus List of Chemical Changes Examples
Question 16-
Classify the following processes into physical or chemical changes-
(i) Beating of aluminium metal to make aluminium foil.
Answer- Beating of aluminium metal to make aluminium foil is a physical change.
Explanation- Beating aluminium metal changes its shape and size, but it does not alter its chemical composition. The substance remains aluminium throughout the process, so it is considered a physical change.
(ii) Digestion of food.
Answer- Digestion of food is a chemical change.
Explanation- Digestion involves the breakdown of complex food molecules into simpler ones through chemical reactions in the body. New substances, such as nutrients, are formed during this process, making it a chemical change.
(iii) Cutting a log of wood into pieces.
Answer- Cutting of a log of wood into pieces is a physical change.
Explanation- Cutting wood changes its size and shape, but it does not change its chemical composition. The substance remains wood throughout the process, so it is considered a physical change.
(iv) Burning of crackers.
Answer- Burning of crackers is a chemical change.
Explanation- Burning crackers involves a chemical reaction between the substances in the cracker and oxygen in the air. New substances are formed, including gases that create the explosion and light. Therefore, it is classified as a chemical change.
Question 17- Write word equations for two chemical reactions with the help of materials given in the box. Air, copper sulphate, iron, vinegar, iron oxide, carbon dioxide, iron sulphate, copper, lime water, water.
Answer 1-
Reaction 1- Materials Used-
- Iron
- Oxygen from the air
Word Equation- Iron + Oxygen → Iron Oxide
Explanation- In this reaction, iron reacts with oxygen from the air to form iron oxide, commonly known as rust. This is a classic example of the rusting of iron.
Reaction 2- Materials Used-
- Vinegar (acetic acid)
- Baking soda (sodium bicarbonate)
Word Equation- Vinegar + Baking Soda → Carbon Dioxide + Water
Explanation- In this reaction, vinegar (which contains acetic acid) reacts with baking soda (sodium bicarbonate) to produce carbon dioxide gas and water. This reaction is commonly used in baking and is responsible for leavening in recipes like making cakes rise.
Question 18(a)- Explain the following- (a) Lime water turns milky on passing carbon dioxide gas into it.
Answer 18(a)- Lime water turns milky when carbon dioxide gas is passed into it due to the formation of calcium carbonate (CaCO3) as a white precipitate. The chemical reaction can be described as follows-
Explanation 18(a)- When carbon dioxide gas (CO2) is introduced into lime water (calcium hydroxide, Ca(OH)2), it reacts with the calcium hydroxide to form calcium carbonate (CaCO3), which is sparingly soluble in water. The formation of calcium carbonate as a solid precipitate causes the lime water to appear milky or cloudy. This reaction is used as a test for the presence of carbon dioxide gas and is commonly seen in laboratory experiments and in nature when carbon dioxide is absorbed by bodies of water like oceans or lakes.
Question 18(b)- Explain the following- (b) Bubbles are produced when acetic acid is added to a solution of sodium hydrogen carbonate.
Answer 18(b)– Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate due to the chemical reaction between the two substances, resulting in the release of carbon dioxide gas. The reaction can be described as follows-
Explanation 18(b)– When acetic acid, which is commonly found in vinegar, is added to a solution of sodium hydrogen carbonate (sodium bicarbonate, NaHCO3), a chemical reaction occurs. Acetic acid (CH3COOH) is an acid, and sodium hydrogencarbonate is a base. When they react, they undergo an acid-base reaction, resulting in the formation of carbon dioxide (CO2) gas, water (H2O), and sodium acetate (CH3COONa). The release of carbon dioxide gas during this reaction is responsible for the production of bubbles. This reaction is commonly observed in cooking and is used to leaven baked goods like cakes and bread, causing them to rise and become fluffy.
19. Give two examples for each of the following cases-
(a) Physical changes which are reversible-
- Melting of Ice- When solid ice (H2O) is heated, it melts to form liquid water. This change is reversible as the water can be frozen back into ice when cooled.
- Boiling of Water- Water (H2O) boils and changes into steam (water vapour) when heated. This change is reversible as steam can be condensed back into liquid water when cooled.
(b) Physical changes which are not reversible-
- Breaking a Glass- When a glass is shattered into smaller pieces, it undergoes a physical change. However, it is not easily reversible to restore the original glass.
- Tearing a Piece of Paper- Tearing a piece of paper changes its shape and size, but it cannot be easily reversed to get back the original, unbroken paper.
(c) Chemical changes-
- Burning of Wood- Wood undergoes a chemical change when it burns. It reacts with oxygen to produce carbon dioxide, water vapour, and ash, resulting in a change in its chemical composition.
- Digestion of Food- In our digestive system, food undergoes a series of chemical changes to break down complex molecules into simpler ones, which can be absorbed by the body. This process involves enzymes and acids, leading to the formation of new substances.
20. Give an example of a chemical reaction for each of the following situations-
(a) A change in colour is observed-
Example- Ripening of Bananas Explanation- When green bananas ripen, they undergo a chemical change. The green chlorophyll pigments in the bananas break down, and new pigments, such as yellow and brown, are formed, resulting in a change in colour.
(b) A gas is evolved-
Example- Mixing Vinegar and Baking Soda Explanation- When vinegar (acetic acid) is mixed with baking soda (sodium bicarbonate), a chemical reaction occurs, leading to the evolution of carbon dioxide gas (CO2). This reaction is commonly observed as bubbles forming when these substances are mixed.
(c) Sound is produced-
Example- Combustion of Fuel in an Engine Explanation- When fuel, such as gasoline or diesel, is burned in an engine, it undergoes a chemical reaction with oxygen from the air. This combustion process not only produces heat but also generates sound due to the rapid expansion of gases and vibrations within the engine.
21. If you leave a piece of iron in the open for a few days, it acquires a film of brownish substance, called rust.
(a) Do you think rust is different from iron?
Answer (a)- Yes, rust is different from iron. Rust is a compound known as iron oxide (Fe2O3) that forms when iron reacts with oxygen and moisture in the presence of air. It has a different chemical composition and properties than pure iron.
(b) Can you change rust back into iron by some simple method?
Answer (b)- Rust cannot be easily changed back into pure iron by a simple method. The conversion of iron to rust is a chemical reaction, and reversing this reaction would require a more complex process, such as reduction, which is not a simple method.
(c) Do you think the formation of rust from iron is a chemical change?
Answer (c)- Yes, the formation of rust from iron is a chemical change. It involves a chemical reaction between iron, oxygen, and moisture, resulting in the formation of a new chemical compound (iron oxide) with different properties than iron. This change is not easily reversible.
(d) Give two other examples of a similar type of change. Examples-
- Corroding Aluminum- Aluminum can undergo a similar type of change when it corrodes. Corrosion of aluminium occurs when it reacts with oxygen in the air, forming aluminium oxide (Al2O3), which is different from pure aluminium.
- Tarnishing of Silver- Silver objects can tarnish over time due to a chemical reaction with sulphur compounds in the air, forming silver sulphide (Ag2S). Tarnish is different in colour and composition from pure silver.
22. A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
(a) What changes do you expect?
Answer (a)- When the iron nail is placed in the copper sulphate solution, a chemical reaction is expected to occur. Over time, the iron nail will undergo a chemical change as it reacts with copper sulphate. The nail will start to change in appearance, and the colour of the solution may also change.
(b) Are these changes chemical in nature?
Answer (b)- Yes, the changes observed in this scenario are chemical in nature. The reaction between iron and copper sulphate results in the formation of new substances, including iron sulphate and copper metal. These changes are not reversible by simple physical means.
(c) Write a word equation for the chemical change, if any.
Answer (c)
Word Equation- Iron (Fe) + Copper Sulfate (CuSO4) → Iron Sulfate (FeSO4) + Copper (Cu)
In this reaction, iron displaces copper from copper sulphate, leading to the formation of iron sulphate and copper metal.
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – Crystallisation – Class 7
Also Check – Difference between Physical and Chemical Change
Also Check – Difference between Physical and Chemical Change
Also Check – Physical Changes Examples – A Student’s Guide
Also Check – Examples of Chemical Changes Explained
Also Check – Reversible and Irreversible Changes- A Guide for Young Learners