NCERT Solutions for Class 7 Science Chapter 5- Physical and Chemical Changes explores key topics from the NCERT Class 7 Science syllabus, focusing on the nature and examples of physical and chemical changes. The article presents innovative teaching techniques and offers detailed solutions to NCERT textbook exercises. This resource serves as a valuable guide for students, teachers, and parents seeking clear and concise explanations in line with the NCERT Class 7 Science curriculum.
Table of Contents
Chapter 5- Physical and Chemical Changes Syllabus Summary-
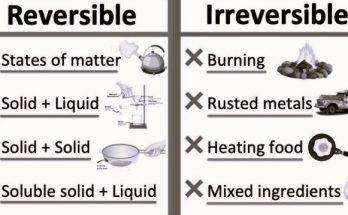
- Physical Changes- Activities demonstrating changes in the physical properties of substances like paper, chalk, and water. These changes do not form new substances and are often reversible.
- Chemical Changes- Understanding changes that produce new substances, such as the rusting of iron.
- Rusting of Iron- Exploring the process of rusting, its effects, and the chemical equation representing rust formation.
- Crystallisation- Learning about crystallisation as an example of a physical change, using activities like forming copper sulphate crystals.
- Key Learnings- Differentiating between physical and chemical changes and understanding their characteristics.
Teaching Methods-
- Interactive Experiments- Perform classroom activities demonstrating physical changes (like melting ice) and chemical changes (like rusting iron).
- Discussion on Everyday Changes- Engage students in identifying and discussing everyday examples of physical and chemical changes.
- Laboratory Activities- Conduct lab experiments like creating copper sulphate crystals to demonstrate crystallisation.
- Practical Applications- Discuss practical applications of these concepts, such as rust prevention in iron.
- Extended Learning Projects- Undertake projects like observing the effect of water on iron nails in different conditions, or researching less polluting cooking fuels.
NCERT Solutions for Class 7 Science Chapter 5- Physical and Chemical Changes – pdf
Chapter 5- Physical and Chemical Changes – Textbook Exercise -(Solved)
1. Classify the changes involved in the following processes as physical or chemical changes:
(a) Photosynthesis
Answer: Chemical Change
Explanation: In photosynthesis, light energy is used to convert carbon dioxide and water into glucose and oxygen. New substances are formed, characterising a chemical change.
(b) Dissolving sugar in water
Answer: Physical Change
Explanation: When sugar is dissolved in water, it merely changes its form from solid to dissolved state. No new substances are formed, indicating a physical change.
(c) Burning of coal
Answer: Chemical Change
Explanation: Burning of coal involves the reaction of carbon in coal with oxygen in the air, forming carbon dioxide. This is a chemical change as new substances are formed.
Also Check – 50 Examples of Physical Changes
(d) Melting of wax
Answer: Physical Change
Explanation: Melting of wax involves only a change in the state of matter from solid to liquid. The molecular structure of wax remains unchanged, signifying a physical change.
(e) Beating aluminium to make aluminium foil
Answer: Physical Change
Explanation: Beating aluminium to make foil changes its shape and thickness but not its composition. No new substances are formed, marking it as a physical change.
(f) Digestion of food
Answer: Chemical Change
Explanation: During digestion, food is broken down into simpler substances like amino acids and glucose through various biochemical reactions, showcasing a chemical change.
2. State whether the following statements are true or false. In case a statement is false, write the corrected statement in your notebook.
(a) Cutting a log of wood into pieces is a chemical change. (True/False)
Answer: False
Corrected Statement: Cutting a log of wood into pieces is a physical change because the wood’s chemical structure is not altered during the cutting process.
(b) Formation of manure from leaves is a physical change. (True/False)
Answer: False
Corrected Statement: Formation of manure from leaves is a chemical change because it involves the decomposition of leaves into new substances (manure) with different chemical properties.
(c) Iron pipes coated with zinc do not get rusted easily. (True/False)
Answer: True
Explanation: Zinc coating (galvanization) protects iron pipes from rusting by preventing their direct exposure to oxygen and moisture, essential factors for rusting.
(d) Iron and rust are the same substances. (True/False)
Answer: False
Corrected Statement: Iron and rust are not the same substances. Rust is iron oxide, formed by the reaction of iron with oxygen and water.
(e) Condensation of steam is not a chemical change. (True/False)
Answer: True
Explanation: Condensation of steam involves a change from the gaseous state to the liquid state, which is a physical change as it does not alter the molecular composition of water.
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
3. Fill in the blanks in the following statements:
(a) When carbon dioxide is passed through lime water, it turns milky due to the formation of _________.
Answer: calcium carbonate (CaCO3)
Completed Statement: When carbon dioxide is passed through lime water, it turns milky due to the formation of calcium carbonate (CaCO3).
(b) The chemical name of baking soda is _________.
Answer: sodium bicarbonate (NaHCO3)
Completed Statement: The chemical name of baking soda is sodium bicarbonate (NaHCO3).
(c) Two methods by which rusting of iron can be prevented are _________ and _________.
Answer: galvanization, painting or greasing
Completed Statement: Two methods by which rusting of iron can be prevented are galvanization and painting or greasing.
(d) Changes in which only _________ properties of a substance change are called physical changes.
Answer: physical
Completed Statement: Changes in which only physical properties of a substance change are called physical changes.
(e) Changes in which new substances are formed are called _________ changes.
Answer: chemical
Completed Statement: Changes in which new substances are formed are called chemical changes.
Also Check – 20 Plus List of Chemical Changes Examples
4. When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Answer: This is a chemical change. When baking soda (a base) is mixed with lemon juice (an acid), a chemical reaction takes place, producing carbon dioxide gas, which is observed as bubbles, and other products like water and salt. This is a neutralisation reaction where the acid and base neutralise each other, resulting in the formation of new substances.
5. When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Answer:
- Physical Change: The melting of the wax is a physical change. As the candle burns, the heat produced causes the solid wax to melt into liquid wax. This is a physical change because it’s a change in the state of matter (from solid to liquid), and no new substance is formed.
- Chemical Change: The burning of the wax is a chemical change. During the burning process, the wax (which is made up of hydrocarbons) reacts with oxygen in the air to produce heat, light, carbon dioxide, and water. This is a chemical change because new substances are formed as a result of the reaction.
- Another Example: Cooking an egg is an example where both physical and chemical changes occur. The egg white and yolk hardening is a chemical change, as new proteins are formed due to the heat. There is also a physical change as the egg changes its form and appearance from raw to cooked.
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
6. How would you show that the setting of curd is a chemical change?
Answer: Setting of curd from milk is a chemical change. This can be demonstrated by considering the following points:
- Formation of New Substance: In the process, milk is transformed into curd, which is a new substance with different properties. The bacteria in the curdling agent (usually a small amount of already prepared curd) ferment the lactose sugar in the milk into lactic acid, which in turn reacts with the proteins in the milk, causing it to curdle.
- Irreversibility: The change from milk to curd is irreversible. Once the milk has turned into curd, it cannot go back to being milk, which is a characteristic of chemical changes.
- Energy Change: There is a slight release of energy in the form of heat during the curdling process, which is common in chemical reactions.
- Change in Composition: The composition of milk changes during the process, with the breakdown of proteins and lactose, which is evident in the change in taste and texture.
Also Check – Difference between Physical and Chemical Change
7. Explain why burning of wood and cutting it into small pieces are considered as two different types of changes.
Answer:
- Burning of Wood: Burning wood is a chemical change. When wood burns, it reacts with oxygen in the air, resulting in the formation of carbon dioxide, water, ash, and releasing heat and light energy. New substances are formed, and the process is irreversible, which are characteristics of a chemical change.
- Cutting Wood into Small Pieces: Cutting wood into small pieces is a physical change. In this case, there’s no new substance formed. The wood is simply being changed into smaller pieces, and its molecular structure remains the same. Physical changes are generally reversible (though in this case it isn’t practically reversible), and there is no change in the internal composition or chemical properties of the wood.
These two processes are different because one involves a change in the internal composition of the wood (chemical change), while the other involves only a change in the physical appearance or form of the wood (physical change).
8. Describe how crystals of copper sulphate are prepared.
Answer: Crystals of copper sulphate (CuSO4) are prepared through the process of crystallisation, which is a physical change. Here is a detailed description of the preparation process:
Preparing a Saturated Solution:
- A beaker is filled with a specific amount of water, and a few drops of dilute sulphuric acid are added.
- The water is heated until it starts boiling.
- Copper sulphate powder is added to the boiling water gradually, with continuous stirring, until no more powder can be dissolved, creating a saturated solution.
Filtration:
The saturated copper sulphate solution is then filtered to remove any undissolved impurities.
Crystallisation:
- The filtered solution is allowed to cool without any disturbance.
- As the solution cools, the copper sulphate starts to crystallise.
- After a while, when you look at the solution, you can observe the blue crystals of copper sulphate forming.
- If crystals do not appear, you may have to wait longer to allow more time for the crystallisation process.
Also Check – What is Crystallisation
9. Explain how painting of an iron gate prevents it from rusting.
Answer: Painting an iron gate is a method used to prevent it from rusting, and this protection occurs due to the following reasons:
Barrier Creation:
- Painting creates a protective barrier between the iron and the environmental factors that cause rusting, such as moisture and oxygen.
Prevention of Moisture Contact:
- Rusting is facilitated by the presence of water or moisture. Paint acts as a sealant that prevents water from coming into direct contact with the iron surface.
Limiting Oxygen Exposure:
- Oxygen from the air is another factor contributing to rusting. A coat of paint limits the amount of oxygen that reaches the iron’s surface, slowing down the oxidation process.
Uniform Coverage:
- A well-painted gate has a uniform coat that doesn’t allow any exposed areas where rusting can initiate.
By painting an iron gate, we effectively reduce the chances of rust formation, helping in maintaining the integrity and appearance of the gate over time.
10. Explain why rusting of iron objects is faster in coastal areas than in deserts.
Answer: Rusting of iron objects is faster in coastal areas compared to deserts due to the environmental conditions that are more conducive to the rusting process.
Presence of Moisture:
- Coastal areas have higher humidity levels due to the proximity to the sea. This means that there is more moisture in the air, facilitating the rusting process as water is one of the essential components for rusting.
Salt Content:
- The sea breeze carries salts, which when deposited on the iron objects, accelerate the rusting process. Salts can increase the conductivity of water, making the electrochemical reactions involved in rusting occur more efficiently.
11. The gas we use in the kitchen is called liquified petroleum gas (LPG). In the cylinder it exists as a liquid. When it comes out from the cylinder it becomes a gas (Change – A) then it burns (Change – B). The following statements pertain to these changes. Choose the correct one.
Options:
(i) Process – A is a chemical change.
(ii) Process – B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer: (ii) Process – B is a chemical change.
Explanation:
- Process – A: In this process, the LPG changes from a liquid to a gas. This is a physical change because it involves a change in state (liquid to gas) and no new substances are formed.
- Process – B: In this process, the LPG burns, reacting with oxygen in the air to produce heat, light, carbon dioxide, and water. This is a chemical change as new substances are formed and energy is released.
12. Anaerobic bacteria digest animal waste and produce biogas (Change – A). The biogas is then burnt as fuel (Change – B). The following statements pertain to these changes. Choose the correct one.
Options:
(i) Process – A is a chemical change.
(ii) Process – B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer: (iii) Both processes A and B are chemical changes.
Explanation:
- Process – A: During this process, anaerobic bacteria digest animal waste, leading to the production of biogas. This involves the breakdown of organic matter by bacteria in the absence of oxygen, resulting in the formation of biogas, which consists mainly of methane and carbon dioxide. This is a chemical change because new substances (biogas) are formed from the initial substances (animal waste).
- Process – B: When biogas is burnt as fuel, it reacts with oxygen in the air, producing heat, carbon dioxide, and water vapour. This process is also a chemical change, as it involves the reaction of methane in the biogas with oxygen, leading to the formation of new substances.
Extended Learning- Activities and Projects
1. Describe two changes that are harmful. Explain why you consider them harmful. How can you prevent them?
Answer:
Harmful Change: Burning of Plastic
- Explanation: When plastic is burned, it releases harmful chemicals such as dioxins, furans, mercury and polychlorinated biphenyls into the atmosphere. These chemicals are dangerous pollutants that can cause significant harm to the environment and living organisms. They can affect human health, causing respiratory issues, and other adverse health effects.
- Prevention: To prevent this harmful change, it is essential to reduce the use of single-use plastics and promote recycling. Communities should be educated about the dangers of burning plastics, and proper waste segregation and disposal methods should be encouraged. Authorities can implement stricter regulations regarding plastic disposal and promote alternative materials.
Harmful Change: Deforestation
- Explanation: Deforestation involves the removal of a vast number of trees and forest lands. This process is harmful because it disrupts ecosystems, contributes to loss of biodiversity, and affects climate regulation. It also leads to the loss of habitat for many species and can disrupt water cycles.
- Prevention: Effective measures to prevent deforestation include enforcing strict regulations against illegal logging, promoting sustainable forestry practices, and encouraging the restoration of degraded lands. Public awareness campaigns can also be beneficial in promoting the conservation of forests and the importance of biodiversity.
Harmful Change: Air Pollution from Vehicle Emissions
- Explanation: The Emissions from vehicles release pollutants such as carbon monoxide, nitrogen oxides, and particulate matter into the air. These pollutants can have severe health impacts, causing respiratory diseases and other health problems. They also contribute to environmental issues like smog and acid rain.
- Prevention: Preventing this harmful change involves promoting the use of public transportation, walking, and cycling to reduce the number of vehicles on the road. Encouraging the use of electric or hybrid vehicles that produce fewer emissions is also crucial. Regular vehicle maintenance and adherence to emission standards can further reduce the pollutants released into the atmosphere.
2. Take three glass bottles with wide mouths. Label them A, B, and C. Fill about half of bottle A with ordinary tap water. Fill bottle B with water which has been boiled for several minutes, to the same level as in A. In bottle C, take the same boiled water and of the same amount as in other bottles. In each bottle put a few similar iron nails so that they are completely underwater. Add a teaspoonful of cooking oil to the water in bottle C so that it forms a film on its surface. Put the bottles away for a few days. Take out nails from each bottle and observe them. Explain your observations.
Answer:
Observations:
Bottle A (Tap Water and Iron Nails):
- The nails in bottle A are likely to show signs of rusting. The oxygen in the water and the surrounding air reacts with the iron, leading to the formation of rust (iron oxide).
Bottle B (Boiled Water and Iron Nails):
- The nails in bottle B will show less rusting compared to those in bottle A. Boiling the water removes dissolved oxygen, which slows down the rusting process, but doesn’t entirely prevent it because some oxygen still remains in the water.
Bottle C (Boiled Water, Iron Nails, and Oil):
- The nails in bottle C will show the least rusting, if any. The oil creates a barrier that prevents oxygen in the air from coming into contact with the water and the nails, further inhibiting the rusting process.
Explanation:
- Rusting is an oxidation reaction where iron reacts with oxygen in the presence of water to form rust (iron oxide).
- In bottle A, ordinary tap water contains more dissolved oxygen, promoting rusting.
- In bottle B, boiling water reduces the amount of dissolved oxygen, which slows down the rusting process.
- In bottle C, the oil layer acts as a protective barrier, preventing or greatly reducing the amount of oxygen that the iron nails are exposed to, minimising rust formation.
Conclusion: This experiment illustrates how the presence of oxygen and water contributes to the rusting process of iron and how removing or reducing these factors can help in preventing or slowing down rusting.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)