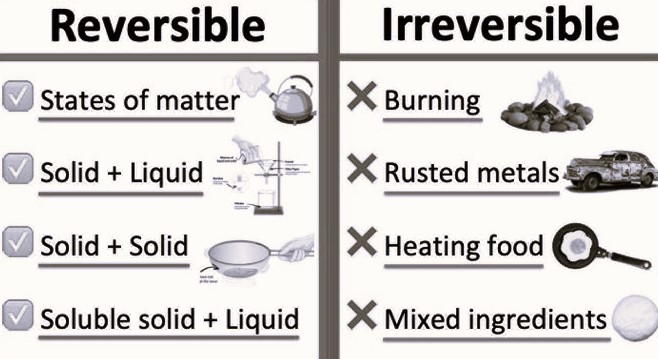
When things around us change, these changes can be of two types- reversible and irreversible. Reversible changes are those we can undo. For example, if we melt ice, it becomes water, and we can freeze it back to ice. This is reversible because we can go back to the starting point.
Irreversible changes are permanent. Once they happen, we can’t go back to how things were before. A good example is cooking food. Once we cook an egg, we can’t change it back to a raw egg.
Reversible and irreversible changes help us know more about how things in our world work. This article will talk more about these changes, giving examples to make it easy for students to understand.
Table of Contents
What Are Reversible Changes?
Reversible changes are those changes where a material or substance can be changed back to its original state. These changes do not create new substances and are often temporary.
A common example of a reversible change is the heating and cooling of water. When ice (water in its solid state) is heated, it melts and becomes liquid water. If you heat this liquid water further, it changes to water vapour, which is water in its gaseous state. When this water vapour cools down, it changes back to liquid water. If you cool the liquid water even more, it freezes into ice again.
Another example is plastic. When plastic is heated at high temperatures, it can be moulded into almost any shape. Once it cools down, it retains the new shape, but its chemical composition remains unchanged. This property of plastic makes it a material that undergoes reversible changes when subjected to different temperatures.
Key Characteristics of Reversible Changes-
- No new substance is formed- The material remains the same chemically, even though its physical state or shape might change.
- Temporariness- These changes are not permanent and can be reversed by altering conditions like temperature.
Everyday Examples of Reversible Changes
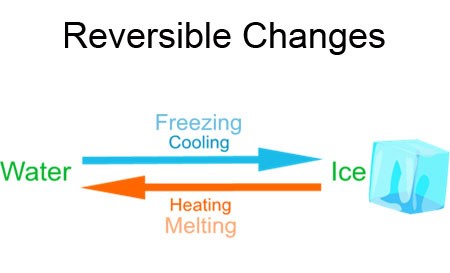
Reversible changes are a part of our daily lives, often occurring without us even noticing. Here are some common examples-
- Melting Butter-
- When you heat butter, it melts from a solid to a liquid. If you cool it down, it solidifies again. This change is reversible because the butter’s chemical composition doesn’t change during melting and solidifying.
- Folding Paper-
- When you fold a piece of paper, its shape changes. If you unfold it, the paper goes back to its original shape, although the crease may still be visible. This is a reversible physical change as the paper’s material properties remain the same.
- Dissolving Sugar in Water-
- When sugar is dissolved in water, it seems to disappear, but it’s just mixed with the water. By evaporating the water, you can get the sugar back in its solid form. The sugar doesn’t change chemically when it dissolves, making this a reversible change.
- Stretching a Rubber Band-
- A rubber band can be stretched and then allowed to return to its original size and shape. This elasticity demonstrates a reversible change as long as the rubber band is not stretched beyond its limit.
- Inflating and Deflating a Balloon-
- When you inflate a balloon, it expands. Once the air is let out, the balloon deflates back to its original size. The balloon doesn’t undergo any chemical change during this process.
- Freezing Water into Ice Cubes-
- Water turns into ice when frozen and melts back to water when heated. This change of state is a typical example of a reversible change.
What Are Irreversible Changes?
Irreversible changes are those where a material or substance undergoes a change that cannot be undone. These changes often result in the creation of new substances.
A simple example is cooking food, like boiling an egg. When an egg is boiled, it changes. The egg’s texture, shape, smell, and appearance transform due to the heat. This change is irreversible; you can’t turn a boiled egg back into a raw egg.
Key Characteristics of Irreversible Changes-
- Permanent Change- Once an irreversible change occurs, it cannot be undone. The original state or form of the material or substance cannot be restored.
- Creation of New Substances- Irreversible changes often lead to the formation of new substances with different properties. For instance, the boiled egg has different characteristics compared to the raw egg.
- Utility of New Materials- The new substances formed from irreversible changes can be useful in different ways, though they are different from the original material.
Real-Life Examples of Irreversible Changes
Irreversible changes are common in our daily lives and understanding them can be quite fascinating. Here are some examples that students can easily relate to-
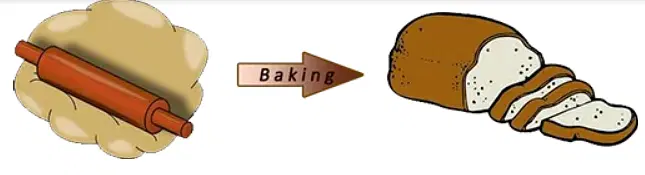
- Baking a Cake-
- When you mix ingredients like flour, sugar, and eggs to bake a cake, the result is a delicious treat. But once baked, you can’t separate these ingredients back to their original forms. This is because baking causes a chemical reaction that changes the ingredients into a new substance.
- Rusting of Iron-
- Rust forms when iron reacts with oxygen in the presence of water or moisture. This process, known as oxidation, changes the surface of the iron into rust. Once rusted, the iron cannot return to its original state without chemical treatment. This is a clear example of an irreversible change.
- Burning Wood-
- When wood burns, it reacts with oxygen in the air and changes into ash, heat, and smoke. This process cannot be reversed, making it an irreversible change. The wood, once burnt, can’t be turned back into its original form.
- Concrete Setting-
- Mixing concrete powder with water creates a thick, hard substance used in construction. Once the concrete has set and hardened, it’s impossible to revert it back to the powder and water state. This solidification is another example of an irreversible change.
Reversible vs. Irreversible Changes- The Differences
When we compare reversible and irreversible changes, we can see clear differences between these two types of processes. Here’s a simple table to illustrate these differences-
Difference Between Reversible vs. Irreversible Changes in Tabular Format
| Aspect | Reversible Process | Irreversible Process |
| Definition | Can be reversed back to original state | Cannot be changed back to original |
| Nature of Change | Temporary | Permanent |
| New Substance Formation | No new substance is formed | New substances are formed |
| Examples | Melting of ice, Folding paper | Cooking, Burning of substances |
Difference Between Reversible vs. Irreversible Changes Explained in Details
Definition
- Reversible Process- This is a change that can be reversed. For example, melting ice can be reversed by refreezing the water back into ice.
- Irreversible Process- This is a change that cannot be undone. Once a substance undergoes an irreversible change, like burning wood to ash, it cannot be returned to its original form.
Nature of Change
- Reversible Process- These changes are usually temporary. The material or substance can easily return to its original state under certain conditions.
- Irreversible Process– These changes are permanent. Once they occur, the original state cannot be recovered.
New Substance Formation
- Reversible Process- No new substance is formed in a reversible process. The material retains its chemical composition even though its physical state might change.
- Irreversible Process- New substances are formed in irreversible processes. These new products have different properties and compositions from the original materials.
Examples
- Reversible Process- Simple actions like dissolving sugar in water, melting ice, or folding dough are examples of reversible processes. They can be undone, returning the material to its original state.
- Irreversible Process- Cooking food, burning substances, or rusting iron are examples of irreversible processes. These result in new substances that are fundamentally different from the starting materials.
Also Check – Difference between Physical and Chemical Change
Also Check – Physical Changes Examples – A Student’s Guide