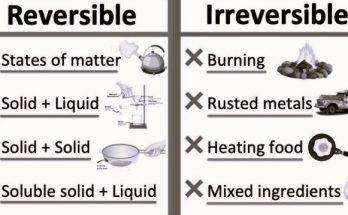
Chapter 6- Physical and Chemical Changes – MCQs (Solved Worksheet)
What is the result of the interaction between iron and copper sulphate solution?
a) Formation of copper sulphate
b) Formation of iron sulphate
c) Formation of a green solution
d) Deposition of iron on copper
Answer: b) Formation of iron sulphate
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
In the reaction between vinegar (acetic acid) and baking soda (sodium hydrogen carbonate), what gas evolved?
a) Oxygen
b) Hydrogen
c) Carbon dioxide
d) Nitrogen
Answer: c) Carbon dioxide
What does the milky appearance of lime water indicate when carbon dioxide is passed through it?
a) Formation of calcium hydroxide
b) Presence of oxygen
c) Presence of carbon monoxide
d) Formation of calcium carbonate
Answer: d) Formation of calcium carbonate
How is rusting prevented in iron objects?
a) By exposing them to open air
b) By limiting exposure to oxygen and moisture
c) By heating them to high temperatures
d) By applying a layer of water
Answer: b) By limiting exposure to oxygen and moisture
What is the outcome of burning magnesium?
a) Formation of magnesium sulphate
b) Formation of magnesium oxide
c) Formation of magnesium hydroxide
d) Formation of magnesium chloride
Answer: b) Formation of magnesium oxide
When iron interacts with copper sulphate solution, what type of reaction occurs?
a) Combination reaction
b) Displacement reaction
c) Acid-base reaction
d) Oxidation-reduction reaction
Answer: b) Displacement reaction
What does the brownish deposit on an iron object exposed to copper sulphate solution indicate?
a) Formation of copper sulphate
b) Formation of iron oxide
c) Deposition of copper
d) Formation of iron sulphate
Answer: c) Deposition of copper
What gas is produced when vinegar reacts with baking soda?
a) Oxygen
b) Hydrogen
c) Carbon dioxide
d) Nitrogen
Answer: c) Carbon dioxide
In the reaction between magnesium and oxygen, what is the product?
a) Magnesium hydroxide
b) Magnesium oxide
c) Magnesium sulphate
d) Magnesium carbonate
Answer: b) Magnesium oxide
What does the hissing sound and bubble formation indicate in the reaction between vinegar and baking soda?
a) Release of oxygen gas
b) Release of carbon dioxide gas
c) Release of hydrogen gas
d) Release of nitrogen gas
Answer: b) Release of carbon dioxide gas
What is the result of exposing iron to open air and moisture?
a) Melting
b) Rusting
c) Evaporation
d) Condensation
Answer: b) Rusting
Which activity demonstrates the reversible nature of phase changes based on temperature adjustments?
a) Cutting a piece of paper
b) Baking bread
c) Reversibility of melting ice
d) Burning paper
Answer: c) Reversibility of melting ice
What process is responsible for the formation of water droplets on the inner surface of an inverted pan held over boiling water?
a) Vaporization
b) Condensation
c) Evaporation
d) Sublimation
Answer: b) Condensation
In the activity involving heating a hack-saw blade, what type of change is observed?
a) Chemical change
b) Biological change
c) Physical change
d) Nuclear change
Answer: c) Physical change
What is the primary composition of rust that forms on iron?
a) Iron
b) Water
c) Oxygen
d) Hydrated iron oxides
Answer: d) Hydrated iron oxides
Which activity involves the transformation of iron into a new substance?
a) Burning paper
b) Reversibility of melting ice
c) Rusting of iron
d) Digesting food
Answer: c) Rusting of iron
What is the defining characteristic of most physical changes?
a) Formation of new substances
b) Change in colour
c) Irreversibility
d) Reversibility
Answer: d) Reversibility
Which concept explains the transformation of a substance from a liquid to a gas due to heating?
a) Vaporization
b) Condensation
c) Evaporation
d) Sublimation
Answer: a) Vaporization
What does the brownish substance formed on the surface of iron exposed to open air and moisture indicate?
a) Physical change
b) Chemical change
c) Biological change
d) Nuclear change
Answer: b) Chemical change
Which activity helps in understanding the nature of substances based on their physical properties?
a) Reversibility of melting ice
b) Rusting of iron
c) Conversion of steam to water droplets
d) Digesting food
Answer: a) Reversibility of melting ice
What is the result of the interaction between iron and copper sulphate solution?
a) Formation of copper sulphate
b) Formation of iron sulphate
c) Formation of a green solution
d) Deposition of iron on copper
Answer: b) Formation of iron sulphate
In the reaction between vinegar (acetic acid) and baking soda (sodium hydrogen carbonate), what gas evolved?
a) Oxygen
b) Hydrogen
c) Carbon dioxide
d) Nitrogen
Answer: c) Carbon dioxide
What does the milky appearance of lime water indicate when carbon dioxide is passed through it?
a) Formation of calcium hydroxide
b) Presence of oxygen
c) Presence of carbon monoxide
d) Formation of calcium carbonate
Answer: d) Formation of calcium carbonate
How is rusting prevented in iron objects?
a) By exposing them to open air
b) By limiting exposure to oxygen and moisture
c) By heating them to high temperatures
d) By applying a layer of water
Answer: b) By limiting exposure to oxygen and moisture
What is the outcome of burning magnesium?
a) Formation of magnesium sulphate
b) Formation of magnesium oxide
c) Formation of magnesium hydroxide
d) Formation of magnesium chloride
Answer: b) Formation of magnesium oxide
When iron interacts with copper sulphate solution, what type of reaction occurs?
a) Combination reaction
b) Displacement reaction
c) Acid-base reaction
d) Oxidation-reduction reaction
Answer: b) Displacement reaction
What does the brownish deposit on an iron object exposed to copper sulphate solution indicate?
a) Formation of copper sulphate
b) Formation of iron oxide
c) Deposition of copper
d) Formation of iron sulphate
Answer: c) Deposition of copper
What gas is produced when vinegar reacts with baking soda?
a) Oxygen
b) Hydrogen
c) Carbon dioxide
d) Nitrogen
Answer: c) Carbon dioxide
In the reaction between magnesium and oxygen, what is the product?
a) Magnesium hydroxide
b) Magnesium oxide
c) Magnesium sulphate
d) Magnesium carbonate
Answer: b) Magnesium oxide
What does the hissing sound and bubble formation indicate in the reaction between vinegar and baking soda?
a) Release of oxygen gas
b) Release of carbon dioxide gas
c) Release of hydrogen gas
d) Release of nitrogen gas
Answer: b) Release of carbon dioxide gas
What is the primary difference between physical changes and chemical changes?
a) Physical changes involve the formation of new substances, while chemical changes do not.
b) Physical changes are always irreversible, while chemical changes are reversible.
c) Physical changes alter the chemical composition, while chemical changes do not.
d) Physical changes alter the form or appearance without changing the chemical composition, while chemical changes transform substances into new ones by altering their chemical composition.
Answer: d) Physical changes alter the form or appearance without changing the chemical composition, while chemical changes transform substances into new ones by altering their chemical composition.
What is the primary product of the reaction when magnesium burns in the presence of oxygen?
a) Magnesium hydroxide
b) Magnesium sulphate
c) Magnesium oxide
d) Magnesium chloride
Answer: c) Magnesium oxide
Which of the following is NOT a manifestation of a chemical change?
a) Burning phenomena
b) Explosive reactions
c) Spoilage of food
d) Melting ice
Answer: d) Melting ice
What happens when iron interacts with copper sulphate solution?
a) Formation of iron sulphate and deposition of copper
b) Formation of copper sulphate and deposition of iron
c) Formation of iron chloride and deposition of copper
d) Formation of copper chloride and deposition of iron
Answer: a) Formation of iron sulphate and deposition of copper
What gas is released when vinegar reacts with baking soda?
a) Oxygen
b) Hydrogen
c) Carbon dioxide
d) Nitrogen
Answer: c) Carbon dioxide
Which of the following is a consequence of rusting?
a) Increased durability of iron objects
b) Decreased maintenance costs in industries
c) Significant economic implications due to damage
d) Enhanced resistance to environmental exposure
Answer: c) Significant economic implications due to damage
What is the result of the neutralisation process?
a) Formation of water and salt
b) Formation of oxygen gas and water
c) Formation of carbon dioxide and water
d) Formation of hydrogen gas and salt
Answer: a) Formation of water and salt
What chemical reaction occurs during rusting?
a) Redox reaction
b) Acid-base reaction
c) Precipitation reaction
d) Complexation reaction
Answer: a) Redox reaction
Which of the following substances is commonly associated with the prevention of rusting in iron objects?
a) Zinc
b) Copper
c) Aluminium
d) Silver
Answer: a) Zinc
What type of reaction occurs when iron displaces copper from a copper sulphate solution?
a) Combination reaction
b) Decomposition reaction
c) Displacement reaction
d) Precipitation reaction
Answer: c) Displacement reaction
What is the purpose of applying a protective coating like paint to iron objects?
a) To make them look more appealing
b) To enhance their electrical conductivity
c) To create a barrier against oxygen and moisture
d) To increase their weight and durability
Answer: c) To create a barrier against oxygen and moisture
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Also Check – Crystallisation – Class 7
Also Check – Difference between Physical and Chemical Change