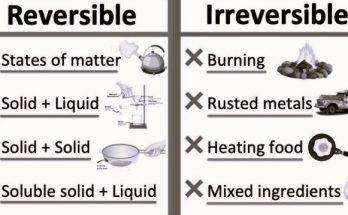
In Class 7 science, the chapter on “Physical and Chemical Changes” covers chemical changes where substances form new materials. This article explains activities like burning magnesium ribbon, reacting copper sulphate with iron, and mixing vinegar with baking soda, showing how these processes create new substances with different properties.
Table of Contents
Activity 5.6- Burning Magnesium Ribbon
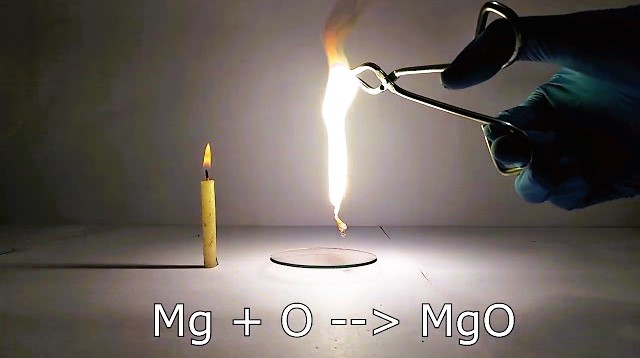
Objective-
To demonstrate a chemical change by burning a magnesium ribbon.
Procedure-
- A small piece of a magnesium ribbon is cleaned and burned.
- The ribbon burns with a brilliant white light and turns into a powdery ash.
- Students are advised not to stare directly at the burning ribbon as it can be dangerous for the eyes.
Concepts Covered-
- Chemical Change- When the magnesium ribbon burns, it reacts with oxygen in the air to form magnesium oxide (MgO), a new substance. This reaction is a chemical change.
- Formation of a New Substance- Unlike physical changes, chemical changes result in the formation of new substances. In this case, magnesium oxide, which is different from the original magnesium ribbon, is formed.
- Properties of the Product- The ash (magnesium oxide) collected after burning is mixed with water to form magnesium hydroxide [Mg(OH)2], which is a base. This shows the change in properties from the original magnesium ribbon.
Learning Outcome-
Students understand that burning is a chemical change that results in new substances with different properties. The experiment highlights the nature of chemical reactions, where the reactants (magnesium and oxygen) form new products (magnesium oxide and magnesium hydroxide) with different chemical properties.
Activity 5.7- Copper Sulphate and Iron

Objective-
To demonstrate a chemical reaction that results in the formation of new substances.
Procedure-
- Dissolve a teaspoonful of copper sulphate in about half a cup of water in a glass tumbler or a beaker.
- Add a few drops of dilute sulphuric acid to the solution.
- Drop a nail or a used shaving blade into the solution and wait for about half an hour.
- Observe the colour change in the solution and any deposits on the iron nail.
Concepts Covered-
- Chemical Reaction- When iron (the nail or blade) is added to the copper sulphate solution, a chemical reaction occurs. Copper sulphate reacts with iron, leading to the formation of iron sulphate and copper.
- Formation of New Substances- The solution’s colour changes from blue to green, indicating the formation of iron sulphate, a new substance. Additionally, a brown deposit of copper forms on the iron nail, signifying another new substance.
- Chemical Equations- This chemical change can be represented by a chemical equation, showing the reactants (copper sulphate and iron) and the products (iron sulphate and copper).
Learning Outcome-
Students learn that in a chemical reaction, the original substances (reactants) change into new substances (products) with different properties. This activity helps them understand the concept of chemical changes and how to identify them through observable changes, such as colour change or the formation of a new substance.
Activity 5.8- Vinegar and Baking Soda

Objective-
To demonstrate a chemical reaction between an acid (vinegar) and a base (baking soda), resulting in the formation of new substances.
Procedure-
- Take a teaspoonful of vinegar in a test tube.
- Add a pinch of baking soda to the vinegar.
- Observe the reaction – you will hear a hissing sound and see bubbles of gas forming.
- Pass this gas through freshly prepared lime water.

Concepts Covered-
- Acid-Base Reaction- Vinegar, which is acetic acid, reacts with baking soda, which is sodium bicarbonate (a base), to produce a chemical reaction.
- Gas Formation- The reaction produces carbon dioxide gas, evidenced by the hissing sound and bubbles.
- Chemical Change Indicators- The formation of gas and the change in the substances’ properties (from solid and liquid to gas) indicate a chemical change.
- Interaction with Lime Water- When the carbon dioxide gas produced is passed through lime water, it turns the lime water milky. This is due to the formation of calcium carbonate, a new substance.
Learning Outcome-
Students learn how an acid reacts with a base to produce a gas, demonstrating a chemical change. They also observe how new substances are formed as a result of chemical reactions, which is a key characteristic of chemical changes.
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Reversible and Irreversible Changes- A Guide for Young Learners
Also Check – Difference between Physical and Chemical Change
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Also Check – Difference between Physical and Chemical Change