
Define Corrosion class 10
Corrosion Corrosion is the type of Oxidation reaction in everyday life in which the open surface of the metals are slowly eaten away by the reaction of air, water and …
Define Corrosion class 10 Read MoreCBSE Class Notes Online – Classnotes123
CBSE Class Notes, Worksheets, Question Answers, Diagrams , Definitions , Diffrence between , Maths Concepts, Science Facts Online – Classnotes123

Corrosion Corrosion is the type of Oxidation reaction in everyday life in which the open surface of the metals are slowly eaten away by the reaction of air, water and …
Define Corrosion class 10 Read MoreQuestion 1 one of the following does not happen during a chemical reaction. This is Breaking of old chemical bonds and formation of new chemical bonds Formation of new substances …
CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS MCQ Read More
Paint is applied on iron articles to shield them from corrosion caused by environmental elements like moisture and oxygen. Corrosion leads to rust formation, tarnishing iron surfaces and compromising their integrity. By forming a protective barrier, paint inhibits rusting, extending the lifespan of iron structures, reducing repair costs, preserving aesthetics, and maintaining structural soundness.
Why do we apply Paint on Iron articles? Read MoreDecomposition reactions are called opposite of combination reactions due to their reverse processes. In a decomposition reaction, compounds break down into simpler substances, contrasting with combination reactions where substances merge to form compounds. For example, heating calcium carbonate (CaCO₃) decomposes it into calcium oxide (CaO) and carbon dioxide (CO₂). This equation demonstrates the reversal of processes in decomposition reactions compared to combination reactions.
Why are decomposition reactions called the opposite of combination reactions? Write an equation for this reaction. Read More
A precipitation reaction occurs when two dissolved substances combine, yielding an insoluble product called a precipitate. Typically, it involves a double displacement reaction where ions switch, forming new compounds. Examples include sodium sulphate with barium chloride, yielding barium sulphate precipitate, and silver nitrate with sodium chloride, forming silver chloride precipitate, crucial in chemical and analytical applications.
What do you mean by a precipitation reaction? Explain by giving examples. Read More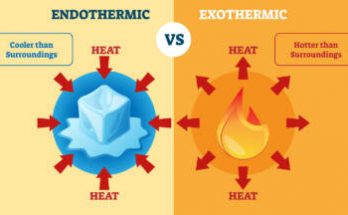
Exothermic reactions release heat, like methane combustion, respiration, carbon burning in oxygen, and organic decomposition. Endothermic reactions absorb heat, such as calcium carbonate decomposition, ferrous sulfate thermal decomposition, silver chloride decomposition, photosynthesis, and electrolysis of water. They require energy input, distinguishing them from exothermic reactions, which release energy.
What does one mean by exothermic and endothermic reactions? Give examples. Read More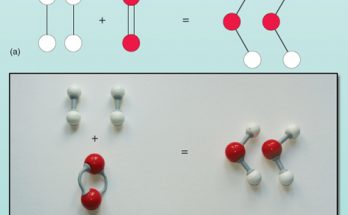
Question: What is a balanced chemical equation? Why should chemical equations be balanced? Answer: A balanced chemical equation is a representation of a chemical reaction where the number of each …
What is a Balanced Chemical Equation? Why should chemical equations be balanced? Read More
Question- Why should a magnesium ribbon be cleaned before burning in air? Answer- A magnesium ribbon is cleaned before burning in air to remove the protective layer of magnesium oxide …
Why should a magnesium ribbon be cleaned before burning in air? Read More