Crystallisation is a fascinating process in the world of chemistry that you’re about to explore in Class 7. This technique allows us to obtain large, pure crystals of substances from their solutions. In simple terms, it’s like growing beautiful, sparkly gems from a liquid mixture! In this article, we will dive into the world of crystallisation, understanding what it is, how it works, and why it’s important.
What is Crystallisation?
Definition
Crystallisation is a physical process that involves the formation of highly organised, well-defined, and pure crystals from a solution. It is a method used to separate a solute (the substance we want as crystals) from a solvent (the liquid it’s dissolved in) by carefully controlling temperature and other conditions.
In Class VI, you learned that common table salt can be obtained by evaporating seawater. However, the salt obtained in this way is not very pure, and the crystals it forms aren’t very clear. Crystallisation, on the other hand, is a method that helps us create large, pure crystals from solutions. It’s like transforming a cloudy liquid into a stunning crystal, which is pure and has a well-defined shape.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
How Does Crystallisation Work?
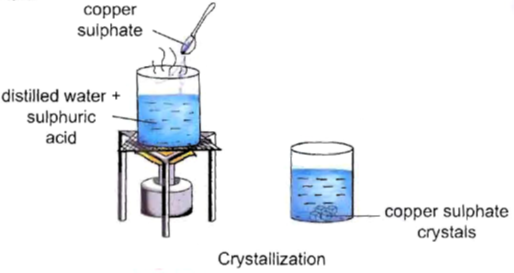
Crystallisation is essentially a “physical change.” It means that we are changing the form of a substance without altering its chemical composition. Here’s a simple experiment to illustrate how it works-
- Materials Needed- You’ll need a cupful of water, a beaker, a few drops of dilute sulfuric acid, and copper sulphate powder.
- Heat the Water- Pour the cupful of water into the beaker and add a few drops of dilute sulfuric acid. Heat the water until it starts boiling.
- Add Copper Sulphate- While the water is boiling, slowly add copper sulphate powder to the mixture while stirring continuously. Keep adding copper sulphate powder until it no longer dissolves.
- Filter the Solution- Once you can’t dissolve any more copper sulphate, filter the solution to remove any undissolved particles. This will give you a clear liquid.
- Allow it to Cool- Now, here comes the magic! Let the clear solution cool down. During this process, the copper sulphate molecules will come together in an organised way and form crystals.
- Observe the Crystals- After some time, you’ll see beautiful crystals of copper sulphate forming in the solution. These crystals are pure and have a specific, recognizable shape.
Why is Crystallisation Important?
Crystallisation is not just a cool experiment; it has real-world applications. Here are a few reasons why it’s important-
1. Purification
Crystallisation helps purify substances. By forming crystals, impurities are left behind in the solution, resulting in a pure product. This is crucial in various industries, from pharmaceuticals to food production.
2. Medicine Production
Many medicines are produced using crystallisation to ensure they are pure and effective. Crystallisation helps in separating the active ingredient from other components in the medicine.
3. Food Industry
In the food industry, crystallisation is used to create familiar items like table sugar. It also helps in achieving the right texture and flavour in candies and chocolates.
4. Chemical Research
Chemists use crystallisation to study the properties of different substances. The size and shape of crystals can provide valuable information about a material’s characteristics.
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Difference between Physical and Chemical Change
Also Check – Physical Changes Examples – A Student’s Guide
Also Check – What is Crystallisation?
Also Check – Examples of Chemical Changes Explained
Also Check – Reversible and Irreversible Changes- A Guide for Young Learners