Physical and Chemical Changes
Definition-
- Physical Change- A transformation that alters the form or appearance of a material without changing its chemical composition.
- Chemical Change- A transformation that turns one material into another by altering its chemical composition.
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Examples of Physical Changes-
- Stretching of a Rubber Band
- Dissolving Sugar in Water
- Crushing of a Can
- Melting Ice
Also Check – 50 Examples of Physical Changes
Examples of Chemical Changes-
- Souring of Milk
- Setting Curd from Milk
- Rusting of Iron
- Burning of Wood
Also Check – 20 Plus List of Chemical Changes Examples
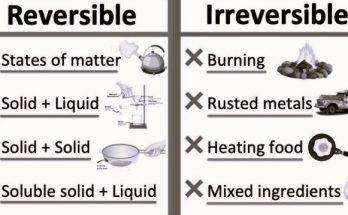
How to Differentiate Physical and Chemical Changes-
- Physical Change Indicators- Change in shape, phase, or size; reversible; no new substance formed.
- Chemical Change Indicators- Change in colour, production of gas, formation of precipitate, temperature change; irreversible; new substances formed.
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Importance of Recognizing Changes-
- Understanding and controlling reactions in cooking, manufacturing, and environmental conservation.
Changes Around You Categorised as Physical or Chemical Changes-
- Physical Changes- Melting ice cream, tearing a piece of paper, boiling water, cutting a tomato, crumpling aluminium foil, freezing water, inflating a balloon, dissolving salt in water, stretching a rubber band, cutting your hair.
- Chemical Changes- Baking bread, digesting food, rusting of iron, burning a candle, photosynthesis in plants, fermentation of grapes to make wine, cooking an egg, ripening of fruits, digesting food, burning paper.
Activity – Cutting a Piece of Paper into Smaller Squares-
- Demonstrated that cutting paper is a physical change that changes the shape and size but does not change the chemical composition or inherent properties of the paper.
- Highlighted that physical changes are often reversible, and properties remain unchanged.
Activity – Chalk from Chalk Dust-
- Showed that chalk dust can be reconstituted back into a solid form (chalk) through physical manipulation.
- Emphasised the concept of reversibility in physical changes.
Activity – Reversibility of Melting Ice-
- Demonstrated the reversibility of the melting process, emphasising that it is an example of a physical change.
- Reaffirmed the concept that physical changes can be reversed without altering chemical composition.
Activity – Conversion of Steam to Water Droplets (Condensation)-
- Showed the process of condensation, where steam turned back into water droplets.
- Highlighted the concept of phase changes influenced by temperature.
Activity – Physical Properties and Physical Changes-
- Illustrated that heating a hack-saw blade changed its colour, indicating a change in its physical property.
- Reinforced the understanding of physical changes and properties.
Chemical Change through Rusting and Chemical Reactions
Concept of Chemical Change-
- Chemical change involves the transformation of one or more substances into new substances with different properties.
Activity Description – Observing Rusting-
- Rusting is a chemical change where iron reacts with oxygen and moisture to form rust (iron oxide).
- Rust is different from the original iron, indicating a new substance is formed.
Key Concepts-
- Environmental Influence- Rusting occurs due to exposure to oxygen and moisture.
- Irreversibility- Unlike physical changes, rusting is irreversible.
- Practical Implications- Rusting can be prevented through methods like painting or galvanising.
Chemical Reactions – Burning of Magnesium-
- Burning magnesium results in the formation of magnesium oxide, showcasing a chemical change.
- The ash formed (magnesium oxide) can further react with water to form magnesium hydroxide.
Chemical Reactions – Copper Sulphate and Iron Interaction-
- Iron displaces copper in copper sulphate, forming iron sulphate and depositing copper on the iron.
- Demonstrates a displacement reaction where a more reactive metal displaces a less reactive one.
Chemical Reactions – Vinegar and Baking Soda Interaction-
- Vinegar and baking soda react to produce carbon dioxide gas.
- The presence of carbon dioxide is confirmed by passing it through lime water, turning it milky.
Chemical Changes and Reactions-
- Chemical changes involve transformations into new substances and are essential in biological, environmental, industrial, and pharmaceutical processes.
- Manifestations of chemical changes include burning, explosive reactions, food spoilage, discoloration in fruits and vegetables, and neutralisation.
Neutralisation-
- Neutralisation is a chemical change as it results in the formation of new substances, namely water and salt.
Rusting, Its Prevention, and Crystallisation
Rusting-
- Rusting is a natural process where iron reacts with oxygen and water to form iron oxide (rust).
- It involves a redox reaction where iron loses electrons and oxygen gains electrons.
- Factors influencing rusting include environmental exposure and humidity.
Prevention Strategies-
- Protective Coatings- Applying paint or grease creates a barrier against oxygen and moisture.
- Galvanization- A zinc layer protects iron by reacting before iron does.
- Stainless Steel- Chromium in stainless steel forms a protective oxide layer, preventing rust.
- Alloying- Mixing iron with other metals or nonmetals creates rust-resistant alloys.
Consequences of Rusting-
- Rusting has economic implications, requiring repair or replacement of infrastructure.
- Industries like shipping incur significant maintenance costs due to rust damage.
Crystallisation-
- Crystallisation is a purification technique forming pure crystals from a saturated solution.
- It results in larger, well-defined crystals, leaving impurities behind in the solution.
- It is a physical change, altering the physical form without changing the chemical identity.
Crystallisation Process-
- Formation of Solution.
- Creation of a saturated solution.
- Cooling and crystal formation.
- Separation of crystals from the solution.
Differences from Evaporation-
- Crystallisation results in pure substances.
- It yields larger and well-defined crystals.
Applications-
- Crystallisation is used in industries like pharmaceuticals and food for purification and solid substance formation.
Crystallisation of Copper Sulphate-
- An experiment illustrating crystallisation.
- Copper sulphate is dissolved, filtered, and allowed to cool to form pure crystals.
- It’s a physical change, changing the physical state without altering the chemical nature of copper sulphate.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – Crystallisation – Class 7
Also Check – Difference between Physical and Chemical Change
Also Check – Physical Changes Examples – A Student’s Guide
Also Check – Examples of Chemical Changes Explained
Also Check – Reversible and Irreversible Changes- A Guide for Young Learners