Table of Contents
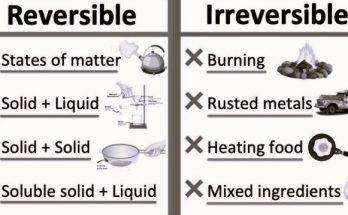
Physical and Chemical Changes
Definition
Physical Change- A transformation that alters the form or appearance of material but does not make the material into another substance.
Chemical Change- A transformation that turns one material into another by altering its chemical composition.
Examples of Physical Changes
- Stretching of a Rubber Band- Temporarily changes shape, but it remains rubber.
- Dissolving Sugar in Water- Sugar particles are dispersed in water, but they can be recovered.
- Crushing of a Can- Changes the shape of the can but not its composition.
- Melting Ice- Ice turns to water, but it remains H2O.
Examples of Chemical Changes
- Souring of Milk- Milk turns sour due to the growth of bacteria, converting lactose into lactic acid.
- Setting Curd from Milk- Bacteria ferment lactose to lactic acid, changing milk’s nature and composition.
- Rusting of Iron- Iron reacts with oxygen and water to form rust, changing its chemical composition.
- Burning of Wood- Wood reacts with oxygen, forming ash, water vapour, and carbon dioxide.
How to Differentiate Physical and Chemical Changes
- Physical Change Indicators- Change in shape, phase, or size, reversible, no new substance formed.
- Chemical Change Indicators- Change in colour, production of gas, formation of precipitate, temperature change, irreversible, new substances formed.
Also Check – 8 Differences between Physical and Chemical Changes
Importance of Recognizing Changes
- Recognizing these changes helps in understanding and controlling reactions better, especially in various applications like cooking, manufacturing, and environmental conservation.
10 changes around you categorised as physical or chemical changes
Physical Changes
- Melting ice cream- When ice cream melts, it undergoes a physical change from a solid to a liquid state.
- Tearing a piece of paper- Tearing paper is a physical change as it changes the shape and size of the paper without forming new substances.
- Boiling water- When water is boiled, it changes from a liquid to a gaseous state, which is a physical change.
- Cutting a tomato- Slicing a tomato is a physical change as it changes the shape and size of the tomato without altering its chemical composition.
- Crumpling a piece of aluminium foil- Crumpling aluminium foil is a physical change that alters the shape and size of the foil.
- Freezing water- The freezing of water is a physical change as it transforms liquid water into solid ice.
- Inflating a balloon- When you inflate a balloon with air, it undergoes a physical change, expanding in size.
- Dissolving salt in water- The dissolution of salt in water is a physical change, as it results in a homogeneous mixture without forming new substances.
- Stretching a rubber band- Stretching a rubber band is a physical change that changes its shape and size.
- Cutting your hair- Trimming or cutting your hair is a physical change, altering the length and shape of your hair strands.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Chemical Changes
- Baking bread- The process of baking bread involves chemical changes, such as the conversion of dough into bread through yeast fermentation.
- Digesting food- Digestion of food in your body is a series of chemical changes that break down complex substances into simpler ones for absorption.
- Rusting of iron- Rusting is a chemical change that occurs when iron reacts with oxygen and moisture in the air, forming iron oxide (rust).
- Burning a candle- The combustion of a candle involves a chemical change, where wax is transformed into carbon dioxide and water vapour.
- Photosynthesis in plants- Photosynthesis is a complex chemical process in plants where carbon dioxide and water are converted into glucose and oxygen using sunlight.
- Fermentation of grapes to make wine- The fermentation of grapes to produce wine is a chemical change involving the conversion of sugars into alcohol.
- Cooking an egg- When you cook an egg, the proteins in the egg white denature and coagulate, resulting in a chemical change.
- Ripening of fruits- The ripening of fruits involves chemical changes, including the breakdown of starches into sugars and changes in flavour and texture.
- Digesting food- Digestion in your stomach is a chemical change, with enzymes breaking down food into nutrients.
- Burning paper- When paper burns, it undergoes a chemical change as the cellulose fibres in paper react with oxygen to produce ash and smoke.
Activity 5.1 -Cutting a piece of paper into smaller squares – Explanation
Task- Cutting a piece of paper into smaller squares and attempting to recreate the original shape by laying them out.
Observation- Even though the paper has been cut into smaller pieces and can’t be rejoined to its original form, the inherent property of each piece (being paper) remains unchanged.
Conclusion- The act of cutting the paper is a physical change because it altered the shape and size but did not change the chemical composition or the inherent properties of the paper.
Key Concepts from Activity 5.1-Cutting a piece of paper into smaller squares
- Physical Changes are often Reversible- Although in this case, the paper can’t be perfectly reverted to its original form, many physical changes (like melting ice into water) can be reversed.
- Properties Remain Unchanged- In physical changes, the inherent properties of the substance remain the same. Here, even after cutting, each piece remains paper with the same texture, colour, and other physical properties.
- Physical Appearance May Change- The size, shape, or state of a substance might change in a physical change, as seen with the cut paper.
Activity 5.2 – Chalk from Chalk Dust-Explanation
Objective of Activity-
- To understand whether chalk can be recovered from its dust through physical manipulation.
Procedure-
- Collection of Chalk Dust- Gather chalk dust from the classroom floor or create dust by crushing a chalk piece.
- Creating a Paste- Mix the chalk dust with water to make a paste.
- Shaping- Roll the paste to form the shape of a chalk piece.
- Drying- Allow the shaped paste to dry.
Conceptual Explanation-
- Physical Change- This activity predominantly involves physical changes. The chalk dust, when mixed with water and reshaped, goes through changes in form and state.
- Reversibility- The chalk dust is essentially compacted back into a solid chalk form, showcasing the reversibility aspect of physical changes.However, it might not be as sturdy or functional as the original piece due to possible impurities and the absence of binders or additives present in manufactured chalk.
Key Learning Points-
- Understanding Reversibility- This activity demonstrates how some materials can revert to their original or near-original form through physical manipulations, emphasising the concept of reversibility in physical changes.
- Exploring Material Properties- The activity allows exploration of the chalk’s properties, understanding its composition, and how it can be manipulated back into a usable form.
Chalk is made from calcium carbonate. Its powdery form can be reconstituted back into a solid form by adding a binding agent (water, in this case), showcasing the flexibility and adaptability of the material.
Activity 5.3- Reversibility of Melting Ice
Objective- To observe the reversibility of the melting process and understand the concept of physical changes.
Procedure-
- Take some ice in a glass or plastic tumbler.
- Place the tumbler in the sun to allow the ice to melt partially.
- You now have a mixture of ice and water in the tumbler.
- Transfer the tumbler to a freezing mixture, which is a mixture of ice and common salt.
Observations-
- Initially, the ice melts in the sun, and you have a mixture of water and ice.
- When the tumbler is placed in the freezing mixture, the water does not become solid ice immediately.
Explanation-
- This activity demonstrates the concept of physical changes. When you melt ice by exposing it to heat (the sun in this case), it changes from a solid (ice) to a liquid (water), but it’s still H2O.
- When you place the tumbler in the freezing mixture, the temperature drops significantly, causing the water to freeze and turn back into solid ice.
- This process is reversible, meaning you can change the state of the substance (from solid to liquid and back to solid) without altering its chemical composition. It’s an example of a physical change.
Activity 5.4- Conversion of Steam to Water Droplets (Condensation)
Objective- Understanding the concept of condensation through boiling water and observing the transformation of steam to water droplets.
Procedure of the Activity-
- Boiling Water- Heat water in a container until it boils and produces steam.
- Collecting Steam- Hold an inverted pan over the steam at a certain distance from the boiling water.
- Observation- Look for any water droplets forming on the inner surface of the inverted pan.
Detailed Explanation-
- Formation of Steam- When water is boiled, it undergoes a physical change from liquid to gas, forming steam. This process is called evaporation or boiling.
- Condensation- Holding a pan over the steam allows the steam to come into contact with a cooler surface. The steam loses heat to the cool surface of the pan and turns back into water droplets, a process known as condensation.
Concepts to Highlight
- Vaporisation- It’s the transformation of a substance from a liquid to a gas due to heating. In this case, water to steam.
- Condensation- This is the process where gas loses heat and turns back into a liquid form. In this case, steam to water droplets.
- Temperature and Phase Changes- The changes between the phases (liquid and gas) are influenced by temperature. Boiling water increases the temperature, causing vaporisation, while a cooler pan causes the steam to lose heat, leading to condensation.
Learning and Observations-
- Understanding Phase Changes- The activity helps understand the reversible nature of phase changes based on temperature adjustments.
- Practical Application- This concept is commonly seen in real-world scenarios such as the water cycle where water evaporates and condenses regularly.
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Physical Properties and Physical Changes
Activity 5.5 Explanation
Procedure
- Using tongs, hold a used hack-saw blade.
- Place the tip of the blade over a gas stove flame.
- Wait for a few minutes and observe the colour change.
- Remove the blade and observe again after some time.
Observations-
- The blade tip changes colour when exposed to the flame.
- After being removed from the flame, the colour of the tip might revert back or change again.
Key Concepts-
- Physical Properties- Attributes like shape, size, colour, and state of a substance.
- Physical Change- A transformation where only the physical properties of a substance alter.
- Reversibility- Most physical changes are reversible, meaning they can be undone or returned to their original state.
Understanding Through Previous Activities
- Activity 5.1 and 5.2- Both highlighted changes in size. Paper was cut, and chalk was broken down, yet the inherent nature of paper and chalk remained unchanged.
- Activity 5.3 and 5.4- Demonstrated water undergoing state changes – from solid ice to liquid water and from steam (gas) back to liquid water.
- Activity 5.5- Showed that the hack-saw blade changed colour due to heating, indicating a change in its physical property.
Detailed Explanation-
- Physical Changes vs. Chemical Changes- Physical changes involve alterations in the form or appearance without changing the substance’s chemical identity. In contrast, chemical changes result in the formation of new substances.
- Importance of Physical Properties- Physical properties help in identifying, distinguishing, and understanding the nature of substances. For example, the state of water (ice, liquid, steam) gives insights about its temperature and energy level.
- Reversibility of Physical Changes- The defining characteristic of most physical changes is their ability to revert to the original state. For instance, melted ice can be refrozen, and condensed steam can be evaporated.
Chemical Change through Rusting

Concept of Chemical Change-
- Chemical change involves a process where one or more substances are transformed into new substances with different properties.
Activity Description- Observing Rusting
- Observation- Leaving iron exposed to open air results in the formation of a brownish substance on its surface known as rust.
- Examples-
- Iron gates, benches, shovels, spades, or iron pans left wet and exposed to the atmosphere undergo rusting.
Also Check – Why do we apply Paint on Iron articles?
Detailed Explanation- Rusting of Iron
- What is Rusting- Rusting is a chemical change where iron reacts with oxygen in the presence of moisture (water) to form rust.
- Composition of Rust- Rust is primarily composed of hydrated iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3). It is different from the iron it forms on, indicating a new substance is created.
Key Concepts-
- Environmental Influence- Exposure to the atmosphere, specifically oxygen and water, facilitates the rusting process.
- Difference from Physical Change- Unlike physical changes, rusting is irreversible and results in the formation of a new substance (rust), which is different from the original material (iron).
Practical Implications-
- Prevention- Rusting can be prevented by limiting the exposure of iron to oxygen and moisture, such as through painting, oiling, or galvanization.
- Impact- Rusting weakens iron objects, diminishing their functionality and lifespan.
Chemical Reactions- The Burning of Magnesium

Objective-Understanding chemical changes by observing the reaction of burning magnesium.
Magnesium Burning- Step-by-Step
- a. Preparation- A magnesium ribbon is taken and cleaned using sandpaper to remove any impurities from its surface.
- b. Ignition- The cleaned magnesium ribbon is brought near a flame, where it catches fire and burns with a bright white light.
- c. Result- After the magnesium ribbon burns completely, it leaves behind a white powdery substance known as magnesium oxide (MgO).
Also Check – Why should a magnesium ribbon be cleaned before burning in air?
Understanding the Change-
- When magnesium burns, it reacts with oxygen from the air, creating a new substance, magnesium oxide. This process is a chemical reaction because a new substance is formed.
Testing the Ash (Magnesium Oxide)-
- a. Mixing- The white powder (ash) left behind is mixed with water. This action leads to another reaction, forming magnesium hydroxide.
- b. Litmus Test- The resulting mixture is tested using blue and red litmus papers. If the mixture is basic, it will turn the red litmus paper blue.
Reactions-
First Reaction-
- Magnesium (Mg) + Oxygen (O2) results in Magnesium Oxide (MgO)
- It is a combustion reaction where magnesium burns in the presence of oxygen.
Second Reaction-
- Magnesium Oxide (MgO) + Water (H2O) results in Magnesium Hydroxide [Mg(OH)2]
- This reaction shows the basic nature of magnesium oxide when mixed with water.
Safety-It is crucial not to look directly at the burning magnesium for a long time, as the bright light produced can be harmful to the eyes.
What did we learn?
- Magnesium undergoes a chemical change when burned, creating a new substance, magnesium oxide. Further reaction with water results in another new substance, magnesium hydroxide, illustrating the concept of chemical changes clearly.
Why is this important?
- Understanding these reactions helps to grasp the concept of chemical changes, where new substances are formed due to reactions, a core concept in chemistry.
Chemical Reactions- Copper Sulphate and Iron Reaction

Objective-Understanding chemical reactions by observing the interaction between copper sulphate and iron.
Reaction Process- Step-by-Step
- Preparation-
- Copper sulphate is dissolved in water.
- A few drops of dilute sulphuric acid are added, yielding a blue solution.
- Observation-
- A small sample of this blue solution is saved for later comparison.
- An iron object (like a nail or blade) is introduced into the main solution.
- The solution is left undisturbed for around thirty minutes.
- Result-
- The blue solution gradually turns green.
- The iron object has a brownish deposit on its surface.
Understanding the Change-
- When iron interacts with the copper sulphate solution, it displaces the copper due to its higher reactivity, leading to the formation of iron sulphate (green solution) and the deposition of copper on the iron object. This is an example of a displacement reaction in chemistry.
Observations
Colour Transformation-
- The initial blue colour of the copper sulphate solution changes to green indicating the formation of iron sulphate.
Copper Deposition-
- The iron object now has a brown coating. This coating is pure copper which has been displaced from the copper sulphate solution by the iron.
Reactions-
Reaction Details-
- Iron + Copper Sulphate Solution (blue) → Iron Sulphate Solution (green) + Copper (brown deposit)
Type of Reaction-
- This is a displacement reaction where a more reactive metal (iron) displaces a less reactive metal (copper) from its salt solution.
Safety-Always wear safety gloves and glasses while conducting the experiment to avoid direct contact with chemicals.
What did we learn?
- The interaction between iron and copper sulphate solution is a classic example of a displacement reaction in chemistry. The iron displaces copper from its solution, leading to a colour change and the deposition of copper on the iron.
Why is this important?
- This experiment vividly demonstrates the reactivity series in metals, where a more reactive metal can displace a less reactive metal from its solution, a foundational concept in chemistry.
Chemical Reactions- Vinegar and Baking Soda Reaction
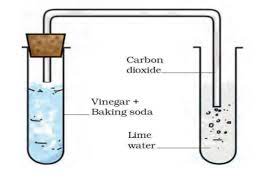
Objective- Understanding the chemical reactions involving vinegar (acetic acid) and baking soda (sodium hydrogen carbonate), and testing the evolved gas using lime water.
Performing the Reaction- Step-by-Step
Preparation-
- Vinegar is measured and placed into a test tube.
- A pinch of baking soda is added to the vinegar.
Observation-
- A hissing sound is heard, and bubbles of gas are observed, indicating the release of a gas.
Collection of Gas-
- The gas evolved through freshly prepared lime water.
Understanding the Change-
- Initial Reaction-
- Vinegar reacts with baking soda, producing carbon dioxide and other substances.
- Testing the Gas-
- The gas, when passed through lime water, turns the lime water milky, indicating the presence of carbon dioxide.
Breaking Down the Reactions-
Vinegar and Baking Soda-
Vinegar (Acetic acid) + Baking soda (Sodium hydrogen carbonate) → Carbon dioxide + other substances
Carbon Dioxide and Lime Water-
Carbon dioxide (CO2) + Lime water [Ca(OH)2] → Calcium Carbonate (CaCO3) + Water (H2O)
What did we learn?
- The reaction between vinegar and baking soda produces carbon dioxide. The presence of carbon dioxide is confirmed by passing it through lime water, which turns milky.
Why is this important?
- Understanding this reaction is essential as it illustrates gas evolution reactions and demonstrates a method for confirming the presence of carbon dioxide.
Chemical Changes and Reactions
Gain a comprehensive understanding of chemical changes through detailed exploration and analysis of various activities, emphasising the formation of new substances and the accompanying characteristics of chemical reactions.
Activity 5.6 – Magnesium’s Reaction with Air-
- When magnesium burns, it reacts with oxygen, resulting in a brilliant white light and leaving behind ash (magnesium oxide).
Activity 5.7 – Iron’s Interaction with Copper Sulphate-
- Iron, when immersed in a copper sulphate solution, instigates a reaction. This reaction leads to the formation of iron sulphate and a copper deposition on the iron surface.
Activity 5.8 – Vinegar and Baking Soda’s Energetic Encounter-
- A lively reaction unfolds when vinegar interacts with baking soda, leading to the release of carbon dioxide. This gas, when passed through lime water, results in a milky solution due to the formation of calcium carbonate.
Chemical Changes-
- Definition- A chemical change is a transformative event where original substances convert into one or more different substances. These transformations are integral to various natural and synthetic processes around us.
Significance of Chemical Changes-
In Biological Processes-
- Digestion, for instance, is a cascade of chemical reactions that breaks down food into absorbable components.
In Environmental Processes-
- Processes like the ripening of fruits or the fermentation of grapes embody chemical transformations fundamental to nature’s rhythm.
In Industrial and Pharmaceutical Arenas-
- The invention of medicines or the synthesis of materials like plastics signifies the application of orchestrated chemical reactions to fulfil human needs.
Manifestations of Chemical Changes
Burning Phenomena
Examples-
- Magnesium Ribbon- When magnesium burns, it undergoes a chemical transformation.
- Common Combustibles- Substances like coal, wood, and leaves, when burned, also undergo chemical changes.
Key Concept-
- Burning is a universal chemical change and is invariably accompanied by the release of heat.
Explosive Reactions
Example- Explosion of fireworks.
- Manifestations- This type of chemical change results in the emission of heat, light, and sound. Furthermore, it releases gases that can have detrimental effects on the atmosphere.
- Safety Note- Due to the potential hazards and environmental implications, it’s recommended to handle fireworks with caution or avoid their use.
Spoilage of Food
Over time, certain foods, when left exposed, emanate a foul odour.
- Interpretation- This unpleasant smell indicates the formation of new substances as a result of chemical reactions, signifying the spoiling of food as a chemical change.
Discoloration in Fruits and Vegetables
- Apple- Slicing an apple and leaving it exposed to air results in the browning of its surface.
- Other Vegetables- Similar observations can be made with slices of potatoes or brinjals (eggplants).
Interpretation-
The change in colour is attributed to the formation of new substances due to chemical reactions with the environment, classifying these transformations as chemical changes.
Neutralisation- Is this process a chemical change?
Answer- Yes, neutralisation is a chemical change because it results in the formation of new substances, notably water and salt.
The Process of Rusting and Its Prevention
What is Rusting?
- Rusting is a natural process that affects iron objects, causing their slow degradation and damage. It’s a common form of corrosion where iron reacts with oxygen and water to form iron oxide, commonly known as rust.
Rusting – Chemical Reaction-
- Rusting involves a redox reaction where iron loses electrons and oxygen gains electrons.
- Equation-
Rusting of Iron-
- Equation- 4Fe + 3O2 + 6H2O → 4Fe(OH)3
- Word Equation- Iron + Oxygen + Water → Iron(III) Hydroxide
Conversion of Iron(III) Hydroxide to Iron(II) Hydroxide-
- Equation- 4Fe(OH)3 → 4Fe(OH)2 + O2 + 2H2O
- Word Equation- Iron(III) Hydroxide → Iron(II) Hydroxide + Oxygen + Water
Conversion of Iron(II) Hydroxide to Iron Oxide-
- Equation- 4Fe(OH)2 → 2Fe2O3 + 3H2O
- Word Equation- Iron(II) Hydroxide → Iron(III) Oxide + Water
Factors Influencing Rusting
Environmental Exposure-
- Iron objects exposed to moist and oxygen-rich environments are more susceptible to rusting.
- The presence of environmental salts, especially in marine environments, accelerates the rusting process.
Humidity-
- Higher humidity levels expedite rust formation due to increased moisture availability for the reaction.
Prevention Strategies
Protective Coatings-
- Applying paint or grease creates a barrier, preventing the iron surface from interacting with oxygen and moisture.
Galvanization-
- A protective zinc layer is added to the iron, preventing rusting. It involves coating iron with zinc, which reacts before iron does, thus protecting the iron.
Advanced Preventive Measures
Stainless Steel-
- Combining iron with carbon and metals like chromium creates stainless steel, an alloy resistant to rusting.
- Chromium in stainless steel forms a protective oxide layer, preventing rust formation.
Alloying-
- Mixing iron with other metals or nonmetals creates alloys that are more resistant to rusting due to altered chemical properties.
Consequences of Rusting
- Rusting has significant economic implications due to the damage it causes to essential infrastructure, requiring repair or replacement.
- In industries like shipping, consistent maintenance and part replacements are necessary, incurring significant costs.
Crystallisation
Crystallisation is a separation and purification technique utilised to form solid crystals from a saturated solution. It results in the formation of pure, well-defined crystals from a solution where impurities may be present.
Process of Crystallisation
1. Formation of Solution-
- Initially, a solution is prepared by dissolving the substance in a suitable solvent. The solution might contain impurities.
2. Saturated Solution-
- The solution is heated, and more solute is added until no more can dissolve, creating a saturated solution.
3. Cooling and Crystal Formation-
- Upon cooling, the solute particles start to separate from the solution, forming crystals. These crystals are more ordered and pure.
4. Separation of Crystals-
- The formed crystals are then separated from the remaining solution, leaving impurities behind in the solution.
Differences from Evaporation
- Purity- Crystallisation results in more pure substances compared to simple evaporation. In crystallisation, impurities are often left behind in the solution.
- Crystal Size and Shape- In crystallisation, larger and well-defined crystals are formed, unlike in evaporation where crystal shape and size might not be as discernible.
Classifying Crystallisation
- Crystallisation is categorised as a physical change. It doesn’t alter the chemical composition of the substance but changes its physical form from a liquid solution to solid crystals.
Applications
- Crystallisation is commonly used in various industries like pharmaceuticals and food for purification and formation of solid substances.
Crystallisation of Copper Sulphate
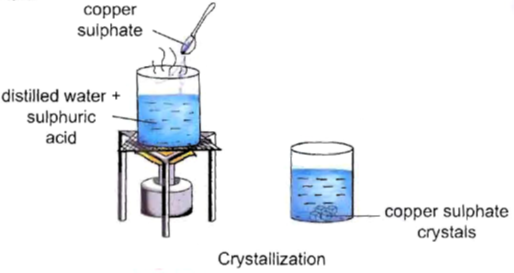
This experiment illustrates the crystallisation process of copper sulphate, a fundamental example of a physical change.
Objective-To prepare crystals of pure copper sulphate from an impure sample.
Materials Needed-
- Water
- Dilute sulphuric acid
- Copper sulphate powder
- Beaker
- Heat source
Procedure-
1. Preparing the Solution-
- Take a cup of water in a beaker.
- Add a few drops of dilute sulphuric acid.
- Heat the water until it starts boiling.
2. Adding Copper Sulphate Powder-
- Add copper sulphate powder slowly while stirring continuously.
- Continue adding until no more powder dissolves.
3. Filtering the Solution-
- Filter the solution to remove any undissolved impurities.
4. Crystallisation-
- Allow the solution to cool without disturbing it.
- Crystals of copper sulphate will form upon cooling.
Analysis and Conclusion-
Observation of Physical or Chemical Change-
- This experiment is primarily a physical change. The copper sulphate dissolves in water and recrystallizes upon cooling.
- Crystallisation doesn’t alter the chemical nature of copper sulphate but changes its physical state.
Key Concepts
- Crystallisation- A purification process where solid crystals form from a saturated solution as it cools.
- Physical Change- A transformation that alters the physical form of a substance without changing its chemical identity.