In Class 7’s science curriculum, the chapter on “Physical and Chemical Changes” introduces students to the concept of physical changes. These are changes where the substance’s identity remains the same, even though its form or appearance might alter. This article delves into activities that clearly illustrate this concept. From cutting paper to boiling water, each activity offers a tangible example of how physical changes occur and can be observed in everyday materials.
Table of Contents
Activity 5.1- Paper Cutting

Objective- To explore whether cutting a piece of paper into smaller pieces changes its properties.
Procedure-
- Start with a whole piece of paper.
- Cut the paper into four equal square pieces.
- Then cut each of these squares into four smaller squares.
- Arrange these pieces to recreate the shape of the original piece of paper.
Concepts Covered-
- Physical Change- This activity demonstrates a physical change. When the paper is cut, its size and shape are altered, but it remains paper. Its basic properties, like colour, texture, and material composition, do not change.
- Irreversibility- Despite the physical change, this process is irreversible. You cannot rejoin the pieces to make the original sheet of paper.
- Understanding of Matter- This activity helps students understand that physical changes can alter the form of a substance without changing its chemical nature.
Learning Outcome:
Students learn that physical changes like cutting alter the appearance or form of a material without changing its chemical composition or properties.
Activity 5.2- Chalk and Water

Objective- To see if chalk can be reformed from its dust.
Procedure-
- Gather chalk dust from the floor or by crushing a piece of chalk.
- Add a little water to this dust to make a paste.
- Shape this paste into the form of a chalk piece and let it dry.
Concepts Covered-
- Physical Change and Reversibility- This activity also illustrates a physical change. The chalk is transformed from solid to powder and then back to a solid form. Unlike paper cutting, this change showcases the reversibility aspect of some physical changes.
- Properties of Materials- The activity helps students understand that some materials can undergo physical changes and still retain their chemical properties. The chalk, even when powdered and reformed, maintains its chemical composition.
Learning Outcome-
Students understand that some physical changes can be reversed, and the material retains its original properties throughout these changes.
Activity 5.3- Ice and Water
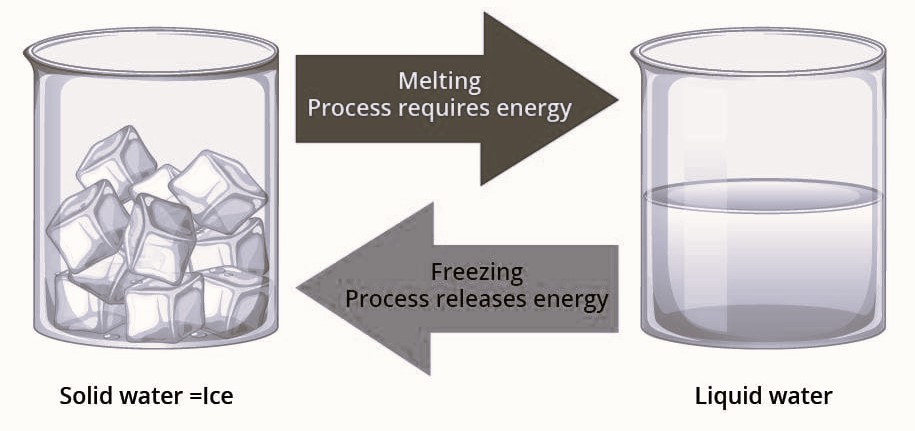
Objective-
To observe the change of state from solid to liquid and back, demonstrating a physical change.
Procedure-
- Place some ice in a glass or plastic tumbler.
- Allow a small portion of the ice to melt by placing the tumbler in the sun, creating a mixture of ice and water.
- Then, place the tumbler in a freezing mixture (ice mixed with common salt).
Concepts Covered-
- Change of State- The ice initially in a solid state melts into liquid water when exposed to the sun, illustrating the change from solid to liquid.
- Reversibility- By placing the mixture in a freezing mixture, the liquid water begins to freeze back into solid ice. This shows the reversible nature of physical changes in state.
- Physical Change- Throughout this process, the chemical composition of water remains the same (H2O), making this a physical change.
Learning Outcome-
Students understand that changes in the state of matter (like melting and freezing) are physical changes and that the substance’s chemical identity remains unchanged.
Activity 5.4- Boiling Water
Objective-
To observe the transition of water from liquid to gas and then back to liquid, a classic example of a physical change.
Procedure-
- Boil water in a container and observe the steam rising.
- Hold an inverted pan over the steam, at a distance from the boiling water.
- Observe the inner surface of the pan for any water droplets.
Concepts Covered-
- Change of State– The boiling water produces steam, showing the transition from liquid to gas.
- Condensation- The steam, when it comes into contact with the cooler surface of the inverted pan, condenses back into water droplets, illustrating the change from gas back to liquid.
- Physical Change- These changes in state (boiling and condensation) do not alter the chemical composition of water. It remains H2O in all three states.
Learning Outcome-
Students learn about the water cycle and the concept of physical changes involving state changes, emphasising that these changes are reversible and do not change the chemical nature of the substance.
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Reversible and Irreversible Changes- A Guide for Young Learners
Also Check – Difference between Physical and Chemical Change
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Also Check – Difference between Physical and Chemical Change