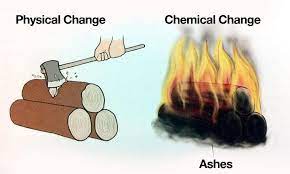
The difference between physical and chemical changes lies at the core of understanding how matter behaves under various conditions.
Table of Contents
9 Key Differences between Physical and Chemical Changes – Tabular Format
| Aspect | Physical Change | Chemical Change |
|---|---|---|
| Definition | A physical change is a change in which the substance’s physical properties (e.g., shape, size, phase) are altered, but its chemical composition remains the same. | A chemical change is a change in which one or more new substances are formed with different chemical compositions. |
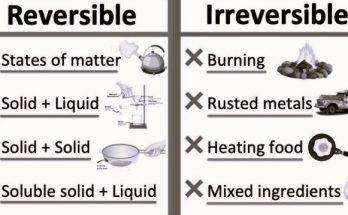
| Reversibility | Physical changes are generally reversible. | Chemical changes are usually not reversible without undergoing another chemical reaction. |
| Formation of New Substances | No new substances are formed in physical changes. | One or more new substances are formed in chemical changes. |
| Change in Chemical Composition | The chemical composition remains unchanged in physical changes. | The chemical composition of substances changes in chemical changes. |
| Examples | Cutting paper, melting ice, boiling water, dissolving sugar in water. | Combustion (burning), rusting of iron, digestion of food, fermentation. |
| Energy Changes | Physical changes may involve energy changes, such as changes in temperature or phase transitions. | Chemical changes typically involve energy changes, such as the release or absorption of heat, light, or sound. |
| Indicators of Change | Physical changes do not usually produce dramatic indicators of change (e.g., colour change, gas production). | Chemical changes often produce indicators like colour change, gas production, or the formation of a precipitate. |
| Reversibility | Physical changes can often be reversed by reversing the conditions that caused them (e.g., freezing water to reverse melting). | Chemical changes are not easily reversible, and reversing them usually requires a different chemical process. |
| Conservation of Mass | In physical changes, the mass of the substance remains the same. | In chemical changes, the mass of the reactants equals the mass of the products, according to the law of conservation of mass. |
Also Chekc – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
9 Differences between Physical and Chemical Changes – Explained in details

Differences between Physical and Chemical Changes in terms of Definition –
Physical Change-
Physical changes refer to alterations in a substance’s physical properties without changing its fundamental chemical composition. These changes are related to the substance’s appearance, state (solid, liquid, or gas), shape, or size. During a physical change, the atoms or molecules that make up the substance remain the same, and no new substances are formed.
For example, when you melt ice, it changes from a solid (ice) to a liquid (water) without altering the chemical identity of water molecules (H2O). Physical changes are often considered more superficial alterations.
Chemical Change-
Chemical changes involve the transformation of substances into new substances with different chemical compositions. These changes are characterised by the rearrangement of atoms or molecules, breaking of chemical bonds, and the formation of new bonds. In a chemical change, the starting materials (reactants) undergo a chemical reaction to produce entirely new substances (products). This process can result in significant changes in properties, such as colour, odour, or the release of energy. Chemical changes are often referred to as chemical reactions and are associated with the formation of new chemical bonds.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Differences between Physical and Chemical Changes in terms of Reversibility-
Physical Change-
Physical changes are typically reversible. This means that you can usually return the substance to its original state by simply reversing the conditions that caused the change.
For example, when you melt ice by applying heat, you create liquid water. By lowering the temperature (removing the heat), you can freeze the water back into ice. Physical changes are often considered temporary alterations since the substance retains its chemical identity throughout the process.
Chemical Change-
Chemical changes are generally not reversible without undergoing another chemical reaction. Once new substances are formed in a chemical reaction, it becomes challenging to return to the original substances. Reversing a chemical change would require a different set of chemical reactions that may not readily occur.
For example, when wood burns (undergoes a chemical change), it produces ash and smoke. Reversing this process to obtain the original wood is not feasible because the chemical structure of the wood has changed, and the ash and smoke are entirely different substances.
Differences between Physical and Chemical Changes in terms of Formation of New Substances-
Physical Change-
In a physical change, no new substances are formed. The original substance remains unchanged chemically, and only its physical properties, such as appearance, state, shape, or size, are altered. This means that the atoms or molecules that make up the substance before and after the change are identical.
For example, when you cut a piece of paper into smaller pieces, you still have paper; you’ve just changed its physical appearance.
Chemical Change-
Chemical changes result in the formation of one or more entirely new substances with different chemical compositions than the original reactants. During a chemical change, the atoms or molecules that made up the reactants rearrange themselves to create new chemical bonds, breaking existing ones. This rearrangement leads to the formation of new compounds or elements.
For example, when iron rusts (undergoes a chemical change), it reacts with oxygen from the air to form iron oxide (rust), which is a completely different substance from the original iron.
Differences between Physical and Chemical Changes in terms of Change in Chemical Composition-
Physical Change-
In physical changes, the chemical composition of the substance remains the same before and after the change. This means that the individual atoms or molecules in the substance are identical in both the initial and final states. Physical changes primarily involve alterations in the substance’s physical properties, like its state (solid, liquid, or gas) or its appearance.
For instance, when you melt ice, the chemical composition remains H2O (water) throughout the process. It changes from a solid state to a liquid state, but the molecules remain unchanged.
Chemical Change-
Chemical changes entail significant changes in the chemical composition of substances involved. The reactants in a chemical reaction undergo transformations, leading to the creation of entirely new substances. This often involves the breaking of chemical bonds in the reactants and the formation of new bonds in the products.
For instance, during the process of combustion (burning), hydrocarbons react with oxygen to produce carbon dioxide and water as the products. In this case, the chemical composition has altered significantly from the reactants to the products.
Differences between Physical and Chemical Changes in terms of Examples-
Physical Change-
Physical changes are alterations in a substance’s physical properties without changing its chemical composition. Here are some examples-
- Cutting Paper- When you cut a piece of paper into smaller pieces, it undergoes a physical change. The paper remains paper; it’s just been divided into smaller segments.
- Melting Ice- As you heat ice, it transitions from a solid state to a liquid state while still being H2O molecules. The chemical composition remains water.
- Boiling Water- Water boils when heated, changing from a liquid state to a gaseous state. Again, it’s still H2O, just in a different physical form.
- Dissolving Sugar in Water- When you add sugar to water, it dissolves, forming a sugar solution. Sugar molecules are dispersed within the water, but their chemical structure remains sugar.
- Changing State of a Substance- Transforming a substance from a solid to a liquid (like melting chocolate) or from a liquid to a gas (like evaporating water) are all examples of physical changes.
Chemical Change-
Chemical changes involve the formation of new substances with different chemical compositions. Here are some examples-
- Combustion (Burning Wood)- When you burn wood, it undergoes a chemical change. Wood reacts with oxygen from the air to produce carbon dioxide, water vapour, and ash, which are entirely different substances from the original wood.
- Rusting of Iron- Iron reacts with oxygen and moisture from the air to form iron oxide (rust). Iron and rust are distinct substances with different properties.
- Digestion of Food in Our Bodies- The process of digesting food involves a series of chemical changes in our stomach and intestines. The food we eat is broken down into simpler substances that can be absorbed by our bodies.
- Fermentation (Production of Yogurt)- During fermentation, microorganisms convert milk sugars into lactic acid, leading to the formation of yoghurt. The milk undergoes a chemical change to become yoghurt.
Differences between Physical and Chemical Changes in terms of Energy Changes-
Physical Change-
Physical changes can involve energy changes, such as alterations in temperature. For example-
- Melting Ice- When you melt ice, it absorbs heat energy from its surroundings to transition from a solid to a liquid. This process is endothermic because it consumes heat energy.
- Freezing Water- The reverse process, freezing water into ice, releases heat energy into the surroundings, making it exothermic.
Chemical Change-
Chemical changes often involve significant energy changes. These can include-
- Combustion (Burning Wood)- When wood burns, it releases a large amount of heat and often produces light and sound, as seen in a campfire. This is an exothermic reaction, as it releases energy into the surroundings.
- Fireworks- The colourful explosions in fireworks displays involve chemical reactions that release heat and produce vibrant light. These are highly exothermic reactions.
- Endothermic Reactions- Some chemical reactions, like certain chemical cold packs used for first aid, absorb heat from their surroundings, leading to a decrease in temperature.
Differences between Physical and Chemical Changes in terms of Indicators of Change-
Physical Change-
Physical changes typically do not exhibit dramatic indicators of change. These changes often involve alterations in a substance’s physical properties, such as its state, size, or shape. Commonly, you won’t observe significant colour changes, gas production, or the formation of solid precipitates in physical changes. For example-
- Dissolving Salt in Water- When you dissolve salt in water, it’s considered a physical change. There’s no change in colour, no gas release, and no solid formation. The salt simply disperses into the water, altering its physical state.
Chemical Change-
Chemical changes often manifest various indicators to signal the transformation. These indicators may include-
- Colour Change- A change in colour can be a clear indicator of a chemical reaction. For instance, when fruits ripen, they often change colour (e.g., bananas turning yellow).
- Gas Production- Many chemical reactions produce gases. A classic example is the reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid), which generates carbon dioxide gas, leading to effervescence and bubbles.
- Formation of Precipitate- In certain chemical reactions, especially double replacement reactions, the formation of a solid precipitate is evident. When two solutions are mixed, and an insoluble substance forms, it appears as a solid settling at the bottom of the container.
Differences between Physical and Chemical Changes in terms of Conservation of Mass-
Physical Change-
In physical changes, the mass of the substance remains unchanged before and after the change. Mass is conserved. This means that the total amount of matter involved in the physical change remains the same. For example-
- Breaking a Glass Bottle- If you take a glass bottle and break it into smaller pieces, the combined mass of all those pieces remains equal to the mass of the original bottle. This principle aligns with the conservation of mass, which asserts that matter is neither created nor destroyed during physical changes.
Chemical Change-
The conservation of mass is a fundamental principle in chemistry that applies to chemical changes. According to this principle, the mass of the reactants (substances before the chemical change) is equal to the mass of the products (substances after the chemical change). In simple terms, matter is neither created nor destroyed in chemical reactions. This law underscores the idea that the total mass remains constant during chemical changes.
Curious Queries – Physical and Chemical Changes
Can a physical change ever become a chemical change?
Answer- No, a physical change involves only the form or state, while a chemical change alters the substance’s composition.
Is cooking food a physical or chemical change?
Answer- Cooking is typically a chemical change because it changes the food’s chemical makeup.
Can a chemical change be partially reversed?
Answer- Some chemical changes can be partially reversed, but it often requires a new chemical reaction.
Why is ripping paper a physical change if it can’t be undone?
Answer– It’s physical because the paper’s chemical composition remains the same, even if the change can’t be physically reversed.
Is dissolving sugar in water a physical or chemical change?
Answer- Dissolving sugar in water is a physical change because it can be reversed (by evaporation) and doesn’t change the chemical composition of the sugar.
If a chemical change is exothermic, can the released energy reverse the change?
Answer– No, the released energy in an exothermic reaction cannot typically reverse the change as it would require a separate chemical process.
If a substance changes colour, is it always a chemical change?
Answer- Usually, a colour change suggests a chemical change, but there are exceptions, like heating metal until it glows red.
Can smashing a crystal be considered a chemical change if it changes shape?
Answer- Smashing a crystal is a physical change. The crystal’s chemical structure remains the same despite its altered shape.
Frequently Asked Questions
What defines a physical change?
Answer- Physical changes alter a substance’s form or state without changing its chemical composition.
What is a chemical change?
Answer- Chemical changes result in the formation of new substances with different chemical properties.
Can physical changes be reversed?
Answer- Most physical changes are reversible, like melting and freezing water.
Are all chemical changes irreversible?
Answer- Chemical changes are generally irreversible, requiring new chemical reactions to reverse.
How do physical and chemical changes differ in energy change?
Answer- Physical changes don’t involve new energy, while chemical changes often release or absorb energy.
What happens to mass in physical and chemical changes?
Answer- Both physical and chemical changes follow the law of mass conservation; mass remains constant.
Do physical changes involve colour changes?
Answer- Physical changes rarely involve colour changes; that’s more typical of chemical changes.
Can a chemical change be identified by smell?
Answer- Often, yes. A new smell can indicate a chemical change, like sulphur’s smell in a reaction.
Is boiling water a physical or chemical change?
Answer- Boiling water is a physical change as it changes state but remains chemically water.
What are signs of a chemical change?
Answer- Signs include colour change, gas production, and temperature change.
Why is burning considered a chemical change?
Answer- Burning transforms substances into entirely new materials, like ash and smoke.Can mixing substances lead to a chemical change?
Answer- Yes, mixing substances can lead to chemical reactions, forming new substances.
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Crystallisation – Class 7
Also Check – Difference between Physical and Chemical Change
Also Check – Physical Changes Examples – A Student’s Guide
Also Check – Examples of Chemical Changes Explained
Also Check – Reversible and Irreversible Changes- A Guide for Young Learners