Chapter 6- Physical and Chemical Changes-Fill in the Blanks with Answers (Solved Worksheet)
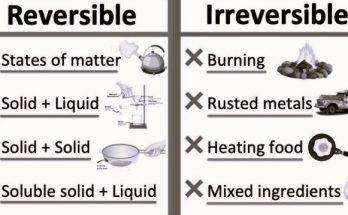
- Cutting a piece of paper into smaller squares is an example of a __________ change. Answer: physical
- In the paper-cutting activity, even though the paper is cut into smaller pieces, the inherent property of each piece, being paper, remains __________. Answer: unchanged
- Physical changes are often __________, meaning they can be undone or returned to their original state. Answer: reversible
- Chalk dust can be collected and used to create a paste, which is then shaped to form a chalk piece. This process is an example of a __________ change. Answer: physical
- Chalk dust, when mixed with water and reshaped, goes through changes in form and state, demonstrating the concept of __________ in physical changes. Answer: reversibility
- In the activity demonstrating the reversibility of melting ice, initially, the ice melts in the sun, changing from a solid (ice) to a liquid (water), which is an example of a __________ change. Answer: physical
- When the tumbler containing the ice and water is placed in a freezing mixture, the water freezes back into solid ice, illustrating the __________ of the melting process. Answer: reversibility
- Boiling water to produce steam is an example of __________, where a substance transforms from a liquid to a gas due to heating. Answer: vaporisation
- The process where gas loses heat and turns back into a liquid form is known as __________, as observed when steam turns into water droplets. Answer: condensation
- In the paper-cutting activity, although the paper is cut into smaller squares, the __________ properties of each piece remain the same. Answer: inherent
- Reversibility is a key concept in understanding __________ changes, where substances can often be returned to their original state. Answer: physical
- Rusting is a natural process that affects iron objects, causing their slow degradation and damage. It’s a common form of __________ where iron reacts with oxygen and water to form iron oxide, commonly known as rust. Answer: corrosion
- Rusting involves a redox reaction where iron loses electrons and oxygen gains electrons, making it a __________ process. Answer: chemical
- The equation for rusting of iron is: 4Fe + 3O2 + 6H2O → __________. Answer: 4Fe(OH)3
- To prevent rusting, one of the methods is applying __________ or grease to create a barrier.Answer: paint
- Galvanization involves coating iron with __________ to prevent rusting.Answer: zinc
- Stainless steel is an alloy that resists rusting because it contains __________, which forms a protective oxide layer.Answer: chromium
- Crystallisation is a separation and purification technique utilised to form solid crystals from a __________ solution.Answer: saturated
- Crystallisation results in the formation of __________, more pure substances with well-defined crystals.Answer: purer
- In crystallisation, impurities are often left behind in the __________.Answer: solution
- Crystallisation is categorised as a __________ change, as it doesn’t alter the chemical composition of the substance.Answer: physical
- When copper sulphate dissolves in water and recrystallizes upon cooling, it undergoes a __________ change.Answer: physical
- The rusting of iron is facilitated by exposure to __________ environments.
Answer: moist - Higher __________ levels expedite rust formation due to increased moisture availability.Answer: humidity
- When iron reacts with copper sulphate solution, it forms __________.Answer: iron sulphate
- In the vinegar and baking soda reaction, the evolved gas is tested using __________, which turns milky in the presence of carbon dioxide.Answer: lime water
- Neutralisation is a chemical change that results in the formation of new substances, notably __________ and salt.Answer: water
- Rusting is a natural process that affects iron objects, causing their slow degradation and damage. It’s a common form of __________ where iron reacts with oxygen and water to form iron oxide, commonly known as rust.Answer: corrosion
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Also Check – Crystallisation – Class 7
Also Check – Difference between Physical and Chemical Change