Plasmids – Structural Details, Functional Roles, and Applications in Genetic Engineering
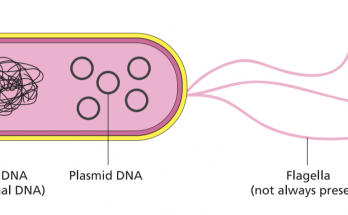
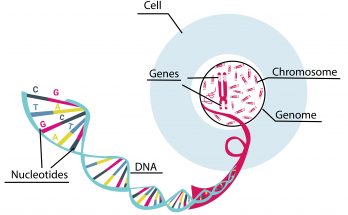
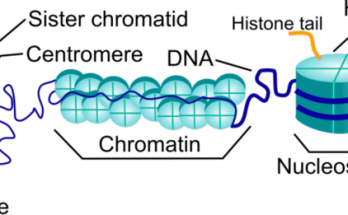
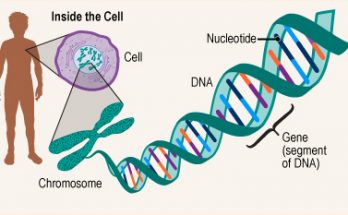
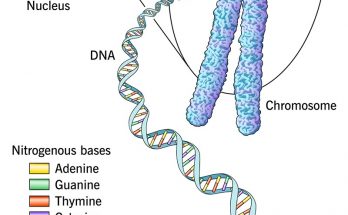
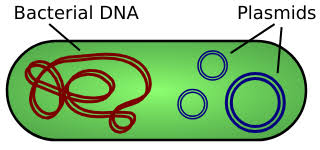
Plasmids are small, circular DNA molecules found in bacteria, separate from chromosomal DNA. They have unique features such as antibiotic resistance and toxin production. Plasmids come in various types like F-plasmids, R-plasmids, and Col plasmids. They replicate independently and facilitate gene transfer through conjugation. Plasmids are also key in genetic engineering and biotechnology.
Plasmids – Structural Details, Functional Roles, and Applications in Genetic Engineering Read More