Table of Contents
Neutralisation
Have you ever wondered what happens when an acid and a base mix? This process is called neutralisation. It sounds complex, but it’s a simple and fascinating concept we encounter in our daily lives. Neutralisation is not just a term in chemistry; it’s a process that helps maintain balance in nature and in many things we use every day. In this article, we will explore what neutralisation is, how it works, and why it’s so important. Whether it’s reducing the acidity in your stomach or helping keep our environment clean, neutralisation plays a crucial role. So, let’s dive into the world of acids, bases, and how they interact to create this balancing act called neutralisation.
Basic Concepts- Acids, Bases, and the pH Scale
Acids and Bases Explained
Before diving into neutralisation, it’s important to understand two key players in this process- acids and bases. Think of acids and bases as opposites in the world of chemistry.
- Acids- These are substances that taste sour (but never taste them in a lab!) and can be corrosive. They turn blue litmus paper red. Examples include lemon juice (citric acid) and vinegar (acetic acid).
- Bases- Bases, on the other hand, are substances that can feel slippery and taste bitter. They turn red litmus paper blue. Examples include baking soda (sodium bicarbonate) and soapy water.
The pH Scale
The pH scale is a way to measure how acidic or basic a substance is. It ranges from 0 to 14-
- Acidic Substances- These have a pH less than 7. The lower the pH, the stronger the acid.
- Basic (or Alkaline) Substances- These have a pH greater than 7. The higher the pH, the stronger the base.
- Neutral Substances- A pH of exactly 7 is neutral, meaning it’s neither acidic nor basic. Pure water is a common neutral substance.
Also Check – A Young Explorer’s Guide to Acids and Bases
Neutralisation Explained
The Balancing Act of Neutralization
Neutralisation is like a dance between acids and bases, where they come together to create harmony. It’s a chemical reaction that occurs when an acid and a base mix, resulting in a balance. But how does this happen? Let’s break it down.
- Acids and Bases Meeting- When an acid (like vinegar) and a base (like baking soda) mix, they don’t just blend; they react. This reaction is the heart of neutralisation.
- Cancelling Out Effects- In this reaction, the properties of the acid and base cancel each other out. The acid loses its acidity, and the base loses its basicity. This means they neutralise each other.
How Neutralization Works
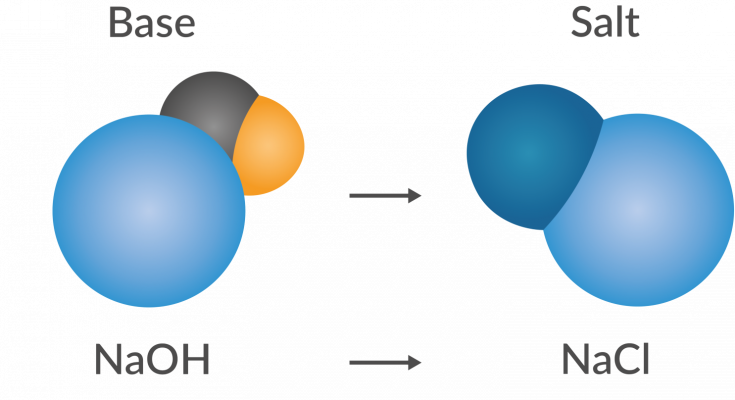
- The Chemical Process- During neutralisation, the hydrogen ions (H+) from the acid combine with the hydroxide ions (OH-) from the base. This union forms water (H2O), a neutral substance.
- Salt Formation- Along with water, neutralisation also produces salt. But this isn’t just table salt; it depends on the acid and base involved. For instance, mixing hydrochloric acid with sodium hydroxide creates sodium chloride (table salt).
Resulting Neutrality
- Achieving Balance- The end product of a neutralisation reaction is neither acidic nor basic; it’s neutral, like pure water. This neutral state is essential in many natural and man-made processes.
Also Check – What is a Neutralisation Reaction?- Explained
Real-World Examples of Neutralization
Neutralisation in Our Daily Lives
Neutralisation is not just a concept in science textbooks; it’s a part of our everyday experiences. Here are some examples where neutralisation plays a key role-
- Antacid Tablets for Stomach Acidity- When you have a stomach ache from too much acidity, antacid tablets can provide relief. These tablets are basic, and they neutralise the excess stomach acid, bringing your stomach back to a comfortable state.
- Neutralizing Bee Stings- Bee stings are acidic. To reduce the sting’s pain, a mild base like baking soda can be applied, which neutralises the acidic sting.
- Cleaning Supplies- Many cleaning agents involve neutralisation. For instance, when you use a vinegar (acidic) and baking soda (basic) mixture for cleaning, a neutralisation reaction occurs, making it effective for removing stains and odours.
Environmental Applications
Neutralization also plays a crucial role in environmental management-
- Neutralising Acidic Soil- In agriculture, lime (which is basic) is often added to acidic soil. This neutralisation helps make the soil more suitable for growing plants.
- Acid Rain Treatment- Acid rain can harm lakes and rivers, making the water too acidic for aquatic life. To counteract this, substances like limestone are added to the water to neutralise the acidity, protecting the ecosystem.
- Industrial Waste Treatment- Before industrial waste is discharged into water bodies, it often undergoes treatment to neutralise harmful acids or bases, ensuring it does not harm the environment.
Also Check – Indicators for Acid and Base- A Guide for Students
Neutralisation- Your Questions Answered
Question- Can neutralisation reactions be reversed?
- Answer- Typically, neutralisation reactions are not reversible. Once an acid and a base have reacted to form water and salt, they usually cannot be turned back into their original forms.
Question- Are all neutralisation reactions safe to observe?
- Answer- While many neutralisation reactions are safe, some involve strong acids and bases and should only be conducted in a lab with proper safety equipment and supervision.
Question- Does neutralisation always produce the same amount of heat?
- Answer- The amount of heat produced in a neutralisation reaction can vary depending on the strength and concentration of the acid and base involved.
Question- How does neutralisation relate to cooking?
- Answer- In cooking, neutralisation is used for flavour balancing. For example, if a dish is too acidic, a cook might add a base like baking soda to neutralise some of the acid.
Question- Why is it important to neutralise industrial waste?
- Answer- Neutralising industrial waste helps prevent damage to the environment and living organisms by balancing the pH and making the waste less harmful.
Question- Can neutralisation be used in medicine?
- Answer- Yes, neutralisation plays a role in medicine, especially in developing antacids that help balance stomach acidity and provide relief from heartburn.
Question- What exactly happens in a neutralisation reaction?
- Answer- In a neutralisation reaction, an acid and a base react to form water and a salt. This reaction essentially cancels out the acidic and basic properties, resulting in a neutral solution.
Question- Can neutralisation be dangerous?
- Answer- Neutralisation is generally a safe reaction, especially in controlled environments like laboratories. However, handling strong acids and bases requires care, as they can be harmful.
Question- Why is neutralisation important in our stomachs?
- Answer- Our stomachs use acid to digest food, but sometimes there’s too much acid, causing discomfort. Neutralisation, like taking an antacid, helps to balance the acid level, providing relief.
Question- Does neutralisation only produce water and salt?
- Answer- Most neutralisation reactions result in water and a type of salt. The exact salt depends on the specific acid and base involved in the reaction.
Question- Can neutralisation help the environment?
- Answer- Yes, neutralisation can be environmentally beneficial. For example, it’s used to treat acidic soil in agriculture and to neutralise acid rain effects in lakes and rivers.
Question- Are there any everyday examples of neutralisation?
- Answer- Absolutely! An everyday example is using baking soda (a base) to neutralise vinegar (an acid) during cleaning. Even our own bodies use neutralisation to manage stomach acidity.
Question- How is neutralisation used in industries?
- Answer- Industries use neutralisation to treat waste products before releasing them into the environment, ensuring they are safe and do not harm ecosystems.
Question- Is neutralisation always a fast reaction?
- Answer- The speed of a neutralisation reaction can vary. Some reactions happen almost instantly, while others take more time, depending on the substances involved.