Chapter 6- Physical and Chemical Changes -Short Questions and Answers(Solved Worksheet)
Table of Contents
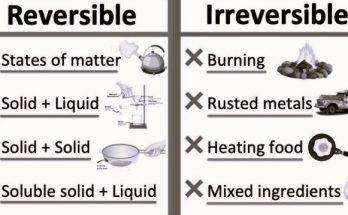
Explain the main difference between a physical change and a chemical change.
Answer: A physical change alters the form or appearance of a substance without changing its chemical composition, while a chemical change transforms one substance into another by altering its chemical composition.
Give an example of a physical change that is not perfectly reversible.
Answer: Melting ice cream.
What are the main indicators of a chemical change?
Answer: Change in colour, production of gas, formation of precipitate, temperature change, and irreversibility are the main indicators of a chemical change.
Why is recognizing physical and chemical changes important?
Answer: Recognizing these changes helps in understanding and controlling reactions better, especially in various applications like cooking, manufacturing, and environmental conservation.
Explain why cutting a piece of paper into smaller squares is considered a physical change.
Answer: Cutting a piece of paper is a physical change because it alters the shape and size of the paper without changing its chemical composition or inherent properties.
Describe the reversibility aspect of physical changes.
Answer: Many physical changes, like melting ice into water, can be reversed, meaning the substance can return to its original state under the right conditions.
What is the result of the fermentation of grapes to make wine?
Answer: The result of the fermentation of grapes to make wine is alcohol.
Why might chalk recovered from its dust through physical manipulation not be as sturdy as the original piece?
Answer: Chalk recovered from its dust through physical manipulation might not be as sturdy as the original piece due to possible impurities and the absence of binders or additives present in manufactured chalk.
What type of change occurs when you cook an egg, and why?
Answer: Cooking an egg involves a chemical change because the proteins in the egg white denature and coagulate, resulting in a change in the chemical composition of the egg.
Explain why rusting of iron is considered a chemical change.
Answer: Rusting of iron is a chemical change because iron reacts with oxygen and moisture in the air to form iron oxide (rust), which is a new substance with a different chemical composition from the original iron.
What happens when you place a mixture of ice and water in a freezing mixture?
Answer: The water in the mixture freezes and turns into solid ice.
Define condensation.
Answer: Condensation is the process where a gas loses heat and turns back into a liquid form.
What are the primary components of rust that form on iron surfaces?
Answer: Hydrated iron oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3).
In the activity involving heating a hack-saw blade, what property changes?
Answer: The colour of the hack-saw blade changes when exposed to heat.
What is the fundamental difference between physical changes and chemical changes?
Answer: Physical changes alter the form or appearance of a substance without changing its chemical composition, whereas chemical changes transform substances into new substances by altering their chemical composition.
What is vaporisation, and in which activity is it demonstrated?
Answer: Vaporisation is the transformation of a substance from a liquid to a gas due to heating. It is demonstrated in the activity of boiling water.
How can you differentiate between a physical change and a chemical change?
Answer: A physical change is reversible and does not alter the chemical composition of a substance, while a chemical change is irreversible and results in the formation of new substances.
What does the formation of rust on iron surfaces indicate about the iron?
Answer: The formation of rust indicates that a chemical change has occurred, and the iron has reacted with oxygen and moisture in the air to form a new substance (rust).
What concept does the activity of converting steam to water droplets (condensation) help us understand?
Answer: It helps us understand the concept of phase changes and the reversibility of these changes based on temperature adjustments.
How is rusting prevented in iron objects, and why is it important?
Answer: Rusting can be prevented by limiting exposure to oxygen and moisture through methods like painting or galvanising. This is important to maintain the functionality and lifespan of iron objects.
What is the primary product of burning magnesium, and why is it considered a chemical reaction?
Answer: The primary product of burning magnesium is magnesium oxide (MgO). It is considered a chemical reaction because a new substance (magnesium oxide) is formed in the process.
How is the presence of carbon dioxide confirmed in the reaction between vinegar and baking soda?
Answer: The presence of carbon dioxide is confirmed by passing it through lime water, which turns milky due to the formation of calcium carbonate (CaCO3).
What is the significance of the interaction between iron and copper sulphate solution?
Answer: This interaction illustrates a displacement reaction, demonstrating that a more reactive metal (iron) can displace a less reactive metal (copper) from its salt solution.
What happens when iron interacts with copper sulphate solution, and what is the result of this interaction?
Answer: When iron interacts with copper sulphate solution, iron displaces copper, resulting in the formation of iron sulphate (green solution) and the deposition of copper on the iron object.
What is the outcome of the reaction between vinegar (acetic acid) and baking soda (sodium hydrogen carbonate)?
Answer: The outcome is the evolution of carbon dioxide gas due to the chemical reaction between vinegar and baking soda.
How is the formation of calcium carbonate confirmed in the reaction with lime water?
Answer: The formation of calcium carbonate is confirmed when carbon dioxide gas is passed through lime water, causing it to turn milky.
Why is understanding rusting prevention important for iron objects?
Answer: Understanding rusting prevention is crucial to maintain the functionality and longevity of iron objects, preventing their deterioration due to corrosion.
What is the main purpose of observing chemical reactions involving vinegar and baking soda?
Answer: The main purpose is to understand gas evolution reactions and confirm the presence of carbon dioxide, demonstrating fundamental concepts in chemistry.
What type of reaction occurs when magnesium burns, and what is the significance of this reaction?
Answer: When magnesium burns, it undergoes a combustion reaction with oxygen, forming magnesium oxide. This reaction is significant as it exemplifies chemical changes, where new substances are formed due to reactions.
Explain why burning magnesium is considered a chemical change.
Answer: Burning magnesium is a chemical change because it involves the transformation of magnesium into a new substance, magnesium oxide, through a chemical reaction with oxygen.
How does the formation of calcium carbonate confirm the presence of carbon dioxide in the reaction between vinegar and baking soda?
Answer: The formation of calcium carbonate confirms the presence of carbon dioxide because when carbon dioxide is passed through lime water, it reacts with calcium hydroxide in lime water to form calcium carbonate, which makes the solution turn milky.
Why is rusting considered a significant concern in industries like shipping?
Answer: Rusting is a significant concern in industries like shipping because it leads to the deterioration of iron-based structures and equipment, requiring consistent maintenance and part replacements, which incur substantial costs.
Describe one method of preventing rusting in iron objects and explain how it works.
Answer: One method of preventing rusting in iron objects is galvanization. It involves coating iron with a protective zinc layer. Zinc reacts with environmental oxygen and moisture before iron does, forming zinc oxide and protecting the iron from corrosion.
What is the primary product of the neutralisation process, and why is it considered a chemical change?
Answer: The primary product of the neutralisation process is water (H2O) and salt (e.g., sodium chloride, NaCl). It is considered a chemical change because it involves the formation of new substances, water and salt, through a chemical reaction between an acid and a base.
How does the interaction between iron and copper sulphate solution demonstrate a displacement reaction?
Answer: The interaction between iron and copper sulphate solution demonstrates a displacement reaction because iron displaces copper from the solution, leading to the formation of iron sulphate and the deposition of copper on the iron surface. This displacement of one element by another is a characteristic of displacement reactions.
What is the significance of understanding chemical changes in biological processes?
Answer: Understanding chemical changes in biological processes is essential because these changes play a crucial role in processes like digestion, where food is broken down into absorbable components through a series of chemical reactions, enabling the body to obtain nutrients.
Explain why the formation of a milky solution when passing carbon dioxide through lime water is a confirmation of the presence of carbon dioxide.
Answer: The formation of a milky solution when passing carbon dioxide through lime water confirms the presence of carbon dioxide because it indicates the formation of solid calcium carbonate (CaCO3) as a result of the reaction between carbon dioxide and calcium hydroxide [Ca(OH)2] in lime water. This change in the solution’s appearance confirms the presence of carbon dioxide.
What is the role of protective coatings in preventing rusting in iron objects?
Answer: Protective coatings, such as paint or grease, create a barrier on the surface of iron objects, preventing direct contact with oxygen and moisture. This barrier inhibits the chemical reaction that leads to rusting, thus preventing corrosion and maintaining the integrity of the iron.
How does alloying with other metals contribute to the prevention of rusting in iron objects?
Answer: Alloying with other metals alters the chemical properties of iron, making it more resistant to rusting. The resulting alloys are less susceptible to the chemical reactions that lead to rust formation, providing enhanced protection against corrosion and rusting.
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
Also Check – Crystallisation – Class 7
Also Check – Difference between Physical and Chemical Change