1. Chemical Change
Table of Contents
Definition-
A chemical change is a process in which one or more substances undergo a transformation, leading to the creation of entirely new substances that possess different properties.
Explanation-
- Chemical changes are characterised by the formation of new substances. During these transformations, the original substances (reactants) are converted into different substances (products).
- Energy changes often accompany chemical changes. This may include the release of heat or light, indicating that chemical bonds within the substances are breaking and forming.
- An illustrative example is the burning of wood. In this process, the wood (composed mainly of cellulose) reacts with oxygen from the air to produce new substances, including carbon dioxide, water, and ash. These products have properties distinct from those of the original wood.
Also Check – Difference between Physical and Chemical Change
2. Chemical Reaction
Definition-
A chemical reaction is a process that involves the reorganisation of molecular or ionic structures within substances, leading to the formation of novel substances.
Explanation-
- A chemical reaction is essentially synonymous with a chemical change. Both terms refer to the same fundamental concept in which substances transform into new substances with different properties.
- In a chemical reaction, reactants are the initial substances that participate in the process. These reactants undergo changes in their molecular or ionic structures to yield products, which are the newly formed substances.
- For instance, when vinegar (acetic acid) reacts with baking soda (sodium bicarbonate), it triggers a chemical reaction. During this reaction, carbon dioxide gas is produced along with other substances as products. This reaction demonstrates the essence of a chemical reaction, where the reactants are modified to generate new chemical entities.
Also Check – Class 7 science -Chapter 6-Physical and Chemical Changes- Complete Notes
3. Crystallisation
Definition-
Crystallisation is a chemical process that involves the formation of solid crystals from a uniform and homogeneous solution.
Explanation-
- Crystallisation is a valuable technique often employed to purify substances. It capitalises on the principle that when a solute (substance to be purified) is dissolved in a solvent and the solution is allowed to cool or evaporate, the solute will start to come together to form ordered, repeating patterns of solid crystals.
- During this process, impurities tend to remain dissolved in the solvent, while the desired substance crystallises out in a more pure form. This makes crystallisation a useful method for obtaining highly pure substances.
- A common example of crystallisation is the production of large, well-defined crystals of pure compounds like copper sulphate. To achieve this, a saturated solution of copper sulphate is prepared, and as it gradually cools, crystals of copper sulphate begin to form, eventually settling at the bottom of the container.
Also Check – Class 7- Chapter 5 – Physical and Chemical Changes – 4 Worksheets Solved and Unsolved
4. Galvanisation
Definition-
Galvanisation is a protective process in which a layer of zinc is applied to the surface of iron or steel to prevent rusting.
Explanation-
- Galvanisation is a corrosion-resistant technique utilised to safeguard iron and steel structures or items from rust and corrosion.
- The process involves coating the surface of iron or steel with a thin layer of zinc. This layer acts as a barrier, preventing the underlying metal from direct contact with environmental factors like oxygen and moisture, which are essential for rust formation.
- Galvanisation is commonly employed in the construction of iron pipes used for carrying water, as these pipes are continuously exposed to moisture. By applying a zinc layer, the pipes are protected against rusting, thus extending their lifespan and ensuring the water remains uncontaminated.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 6- Physical and Chemical Changes
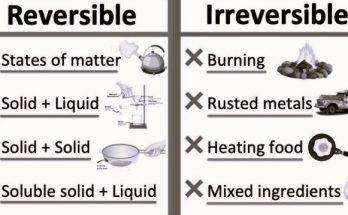
5. Physical Change
Definition-
A physical change is a transformation that affects the physical properties of a substance without altering its chemical composition.
Explanation-
- Physical changes primarily involve alterations in the appearance or characteristics of a substance, such as its shape, size, or state (solid, liquid, or gas). Importantly, no new substances are created during these changes.
- A classic example of a physical change is the melting of ice into water. In this process, the state of water changes from a solid (ice) to a liquid (water), but the chemical composition, which is H2O (water molecules), remains the same. Similarly, freezing water back into ice is another example of a reversible physical change.
Also Check – Difference between Physical and Chemical Change
6. Rusting
Definition-
Rusting is a chemical reaction in which iron reacts with oxygen and moisture from the environment to form rust, which is primarily iron oxide.
Explanation-
- Rusting is a detrimental process that leads to the degradation of iron and steel objects. It occurs when iron comes into contact with atmospheric oxygen (O2) and moisture (usually water vapour). The result is the formation of rust, a reddish-brown, flaky substance.
- This chemical reaction is detrimental to iron-based structures and objects, including bridges, ships, cars, and various metal tools. Rusting can weaken these items, cause structural damage, and lead to costly repairs and replacements.
- The chemical reaction involved in rusting is represented as follows- Iron (Fe) + Oxygen (O2) + Water (H2O) → Rust (Iron Oxide, Fe2O3)
- Rusting is a common form of corrosion and remains a significant challenge, causing substantial economic losses worldwide.
Also Check -Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (MCQs)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Long)
Also Check – Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions for Class 7- Science -Chapter 6- Physical and Chemical Changes
Also Check – Reversible and Irreversible Changes- A Guide for Young Learners