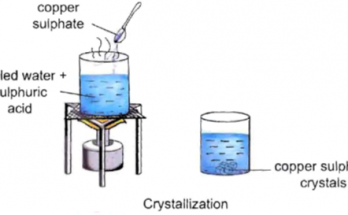
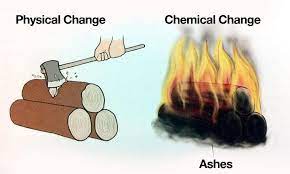
Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short)
Chapter 6- Physical and Chemical Changes -Short Questions and Answers(Solved Worksheet)
Chapter 6- Physical and Chemical Changes-Class 7-Class 7 Question and Answer (Short) Read More