Activity 4.2 in the chapter “Acids, Bases, and Salts” introduces students to the concept of natural indicators in chemistry, specifically using turmeric. This activity is designed to demonstrate how turmeric, a common kitchen spice, can be used to identify basic substances.
Class 7 – Chapter 4- Acids, Bases, and Salts- An Overview of Activities
Activity 4.2- Turmeric as a Natural Indicator
Materials Needed
- Turmeric powder
- Water
- Blotting paper or filter paper
- Soap solution
- Other test solutions (optional)
Preparation of Turmeric Indicator-
- Materials Used- Turmeric powder, water, blotting paper/filter paper.
- Procedure-
- Make a paste using turmeric powder and water.
- Create turmeric paper by depositing the paste on blotting paper/filter paper and allowing it to dry.
- Cut into thin strips for use.
Testing Procedure-
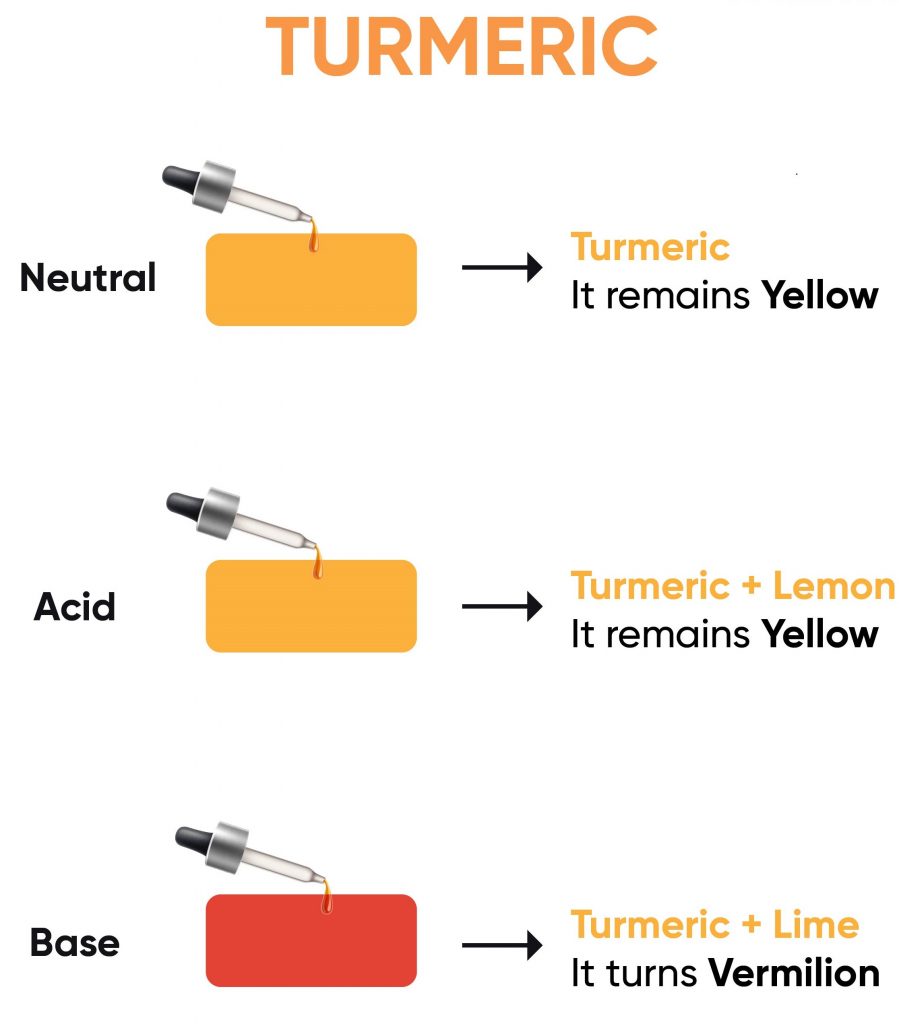
- Objective- To test the effect of various solutions on turmeric paper.
- Method- Place a drop of different test solutions on the strips of turmeric paper and observe any changes.
Table of Observations-
| S. No. | Test Solution | Effect on Turmeric Solution | Remarks |
|---|---|---|---|
| 1 | Lemon Juice | No change | Acidic – No effect on turmeric paper |
| 2 | Orange Juice | No change | Acidic – No effect on turmeric paper |
| 3 | Vinegar | No change | Acidic – No effect on turmeric paper |
| 4 | Milk of Magnesia | Turns red or brownish-red | Basic – Changes colour of turmeric paper |
| 5 | Baking Soda | Turns red or brownish-red | Basic – Changes colour of turmeric paper |
| 6 | Lime Water | Turns red or brownish-red | Basic – Changes colour of turmeric paper |
| 7 | Sugar | No change | Neutral – No effect on turmeric paper |
| 8 | Common Salt | No change | Neutral – No effect on turmeric paper |
Explanation and Remarks-
- Turmeric Paper Reaction- Turmeric paper changes colour when it comes into contact with basic (alkaline) substances.
- Acidic Solutions- Such as lemon juice, orange juice, and vinegar, might not show a significant colour change on turmeric paper as they are not basic.
- Basic Solutions- Such as baking soda and lime water, are expected to change the colour of the turmeric paper, indicating their basic nature.
- Neutral Solutions- Such as sugar and common salt, might not show a colour change as they are neither acidic nor basic.
Also Check – Indicators for Acid and Base- A Guide for Students
Conceptual Understanding
- Natural Indicators-
- Turmeric is a natural indicator, changing colour in the presence of basic substances. It remains yellow with acids or neutral substances but turns red or brown when in contact with a base.
- Understanding Bases-
- This activity primarily focuses on identifying basic substances. Soap solution, being basic, will change the colour of the turmeric paper, while acids or neutral substances will not.
- pH Sensitivity-
- Turmeric, like litmus, is sensitive to the pH of the solution. pH is a scale that measures how acidic or basic a substance is. Turmeric’s colour change is a visual representation of the pH value of the substance it comes in contact with.
- Recording Observations-
- Students should record the initial and final colour of the turmeric paper after applying different solutions. This will help in understanding the nature of the substances tested.
- Safety and Cleanliness-
- Ensure to work in a clean area and wash hands after handling turmeric, as it can stain.
Learning Outcomes
- Identifying Basic Substances- Students learn how to identify basic substances using a natural indicator.
- Chemical Reactions- Understanding how a change in colour indicates a chemical reaction between the turmeric and the substance being tested.
- Application of Natural Resources- The activity emphasises the use of natural, everyday materials in scientific experiments, promoting resourcefulness and creativity.
- Real-world Relevance- Students understand the practical application of chemistry in everyday life, observing how common items like turmeric can be used for scientific purposes.
Conclusion
Activity 4.2 is an excellent experiment for students to understand the basic nature of substances using turmeric, a natural indicator. It demonstrates how simple kitchen ingredients can be transformed into scientific tools, providing hands-on experience in identifying chemical properties of substances. This activity not only reinforces the concepts of acids, bases, and pH but also encourages students to explore and appreciate the wonders of chemistry in everyday life.
Also Check- Young Explorer’s Guide to Acids and Bases
Also Chekc – Rapid Revision – Class 7 Science – Chapter 5-Acids, Bases and Salts
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 5- Acids, Bases and Salts
Also Check – NCERT Solutions For Class 7 Science Chapter 5 – Acids, Bases and Salts
Also Check – Class 7- Chapter 4 – Acids, Bases and Salts – 4 Worksheets Solved and Unsolved
Class 7 science -Chapter 5-Acids, Bases and Salts – Definition and Explanation of Important Keywords
Also Check -Class 7 science -Chapter 5-Acids, Bases and Salts- Complete Notes
