Table of Contents
1. Acid
- Definition- Acids are substances that taste sour and turn blue litmus paper red. They release hydrogen ions (H+) when dissolved in water.
- Examples-
- Citric Acid- Found in citrus fruits like oranges and lemons, used in food and beverages for a sour taste and as a preservative.
- Acetic Acid- Main component of vinegar, used in cooking, baking, and preserving food due to its antibacterial properties.
Properties- Acids have a sour taste and react with most metals to produce salt and hydrogen gas. Their acidic nature makes them excellent preservatives as they inhibit the growth of bacteria and fungi.
Also Check – Class 7 science -Chapter 5-Acids, Bases and Salts- Complete Notes
2. Acidic
- Definition- Refers to the nature of substances that contain acid.
- Explanation- An acidic substance has a pH less than 7. It has a sour taste, and it can neutralise bases. Acidic substances are reactive with metals and can release hydrogen gas.
3. Base
- Definition- Bases are substances that feel slippery to the touch and turn red litmus paper blue. They release hydroxide ions (OH-) when dissolved in water.
- Examples-
- Sodium Hydroxide- A strong base used in soaps and detergents, known for its ability to break down grime and grease.
- Magnesium Hydroxide- Found in milk of magnesia, serves as an antacid and laxative, neutralising stomach acid and relieving heartburn.
Properties– Bases are known for their bitter taste and slippery texture when dissolved in water. They are used in cleaning agents due to their ability to neutralise acids and dissolve oils and in medicine as antacids due to their ability to counteract stomach acid.
4. Basic
- Definition- Refers to the nature of substances that contain a base.
- Explanation- Basic substances have a pH greater than 7. They feel soapy or slippery and are capable of neutralising acids. For example, soap is basic because it contains bases like sodium hydroxide.
5. Indicator
- Definition- Indicators are substances that change colour when exposed to acidic or basic solutions. They are used in chemistry to identify the nature of various substances.
- Examples-
- Litmus Paper- Blue litmus paper turns red in acidic solutions, and red litmus paper turns blue in basic solutions.
- Turmeric- Changes from yellow to red in the presence of a base.
China Rose Petals- Shift from pink in acidic solutions to green in basic solutions.
Also Check – Indicators for Acid and Base- A Guide for Students
Also Check- Young Explorer’s Guide to Acids and Bases
6. Neutral
- Definition- A neutral substance is neither acidic nor basic. In the context of acid-base reactions, neutral refers to a balanced state where neither acidic nor basic properties dominate.
Example- Pure water is a classic example of a neutral substance, with a pH of 7, indicating neither acidic nor basic characteristics.
Also Check – Rapid Revision – Class 7 Science – Chapter 5-Acids, Bases and Salts
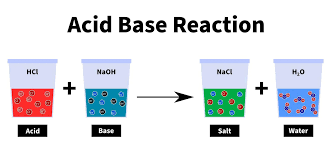
7. Neutralisation
- Definition-Neutralisation is a chemical reaction where an acid and a base react with each other to form salt and water, effectively neutralise the acidic and basic properties of the reactants.
- Practical Examples-
- Treating bee stings with baking soda (a base) neutralises bee venom (acidic).
- Antacids (basic) neutralise excess stomach acid in indigestion cases.
Also Check – What is Neutralisation? – The Basics Explained
Also Check – What is a Neutralisation Reaction?- Explained
8. Salt
- Definition- Salts are versatile compounds formed through the neutralisation reaction between an acid and a base. They have diverse structures and properties.
- Formation- During neutralisation, the hydrogen ions from the acid and the hydroxide ions from the base combine to form water, while the remaining ions form the salt.
- Examples-
- Sodium Chloride (NaCl)- Commonly known as table salt, used in cooking, food preservation, de-icing roads, and water softening.
Also Check – Class 7- Chapter 4 – Acids, Bases and Salts – 4 Worksheets Solved and Unsolved
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 5- Acids, Bases and Salts
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (Long)
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions For Class 7 Science Chapter 5 – Acids, Bases and Salts
Also Check – Class 7 science -Chapter 5-Acids, Bases and Salts- Complete Notes
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (Fill in the Blanks)
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (MCQ)
Class 7 – Chapter 4- Acids, Bases, and Salts- An Overview of Activities