Table of Contents
Acids-
- Definition- Substances that taste sour and turn blue litmus paper red.
- Ion release- Release hydrogen ions (H+) in water.
- Examples- Lemon juice (citric acid), Vinegar (acetic acid).
- Daily Significance- Provide sour taste in foods and drinks.
Bases-
- Definition- Substances that feel slippery and turn red litmus paper blue.
- Ion release- Release hydroxide ions (OH-) in water.
- Examples- Baking soda (used in cooking and cleaning), Soap (removes grease).
- Daily Significance- Neutralise odours, stains, and used in cleaning.
Salts-
- Formation- Result from the reaction of an acid with a base (neutralisation).
- Properties- Ionic compounds, neither acidic nor basic.
- Examples- Table salt (sodium chloride).
- Daily Significance- Used in cooking, food preservation.
Also Check – Rapid Revision – Class 7 Science – Chapter 5-Acids, Bases and Salts
Properties of Acids
- Characteristics of Acids- Acids are one of the most interesting and widely recognized groups of chemical compounds due to their distinct properties and widespread use in various fields, including food, industry, and health.
- Sour Taste- One of the most notable properties of acids is their sour taste. This characteristic is easily noticeable in foods and drinks. Citrus fruits like lemons and oranges are rich in citric acid, contributing to their tangy flavour. Similarly, vinegar, a common household ingredient, owes its sharp taste to acetic acid.
- Reaction with Metals- Acids react with most metals to produce a salt and hydrogen gas. This reaction is not only fundamental in understanding chemical interactions but also has practical applications. For instance, it’s the basis for producing hydrogen gas in laboratories and industries.
Examples of Natural Acids-

Acids Found in Everyday Substances
1. Acetic Acid
- Found in- Vinegar
- Explanation- Acetic acid gives vinegar its acidic taste. It is a key component in vinegar and is used as a preservative and flavoring agent in various culinary applications.
2. Formic Acid
- Found in- Ant’s sting
- Explanation- Formic acid is present in the venom of ant stings. It is responsible for the irritation and pain experienced when an ant bites.
3. Citric Acid
- Found in- Citrus fruits such as oranges, lemons, etc.
- Explanation- Citric acid is naturally present in citrus fruits. It gives these fruits their characteristic tart taste.
4. Lactic Acid
- Found in- Curd (Yogurt)
- Explanation- Lactic acid is formed during the fermentation of milk into curd. It gives curd its tangy flavor.
5. Oxalic Acid
- Found in- Spinach
- Explanation- Oxalic acid is found in vegetables like spinach. However, in large amounts, it can interfere with the absorption of certain nutrients like calcium.
6. Ascorbic Acid (Vitamin C)
- Found in- Amla, Citrus fruits
- Explanation- Ascorbic acid, also known as Vitamin C, is essential for various body functions including the maintenance of skin, blood vessels, bones and cartilage, and wound healing.
7. Tartaric Acid
- Found in- Tamarind, grapes, unripe mangoes, etc.
- Explanation- Tartaric acid is naturally found in plants like tamarind and grapes. It’s often used to give a sour taste to foods and beverages.
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 5- Acids, Bases and Salts
Properties of Bases
- Identifying Characteristics of Bases- Bases are chemical compounds with distinct properties that differentiate them from acids and other substances. Their role in everyday products and industrial applications is significant due to these unique characteristics.
- Bitter Taste and Slippery Feel- Bases are known for their bitter taste and a slippery texture when dissolved in water. This slippery feel is a result of the soap-like substances that form when bases interact with oils and fats, a property utilised in various cleaning products.
Examples of Natural Bases-
Bases Found in Everyday Substances
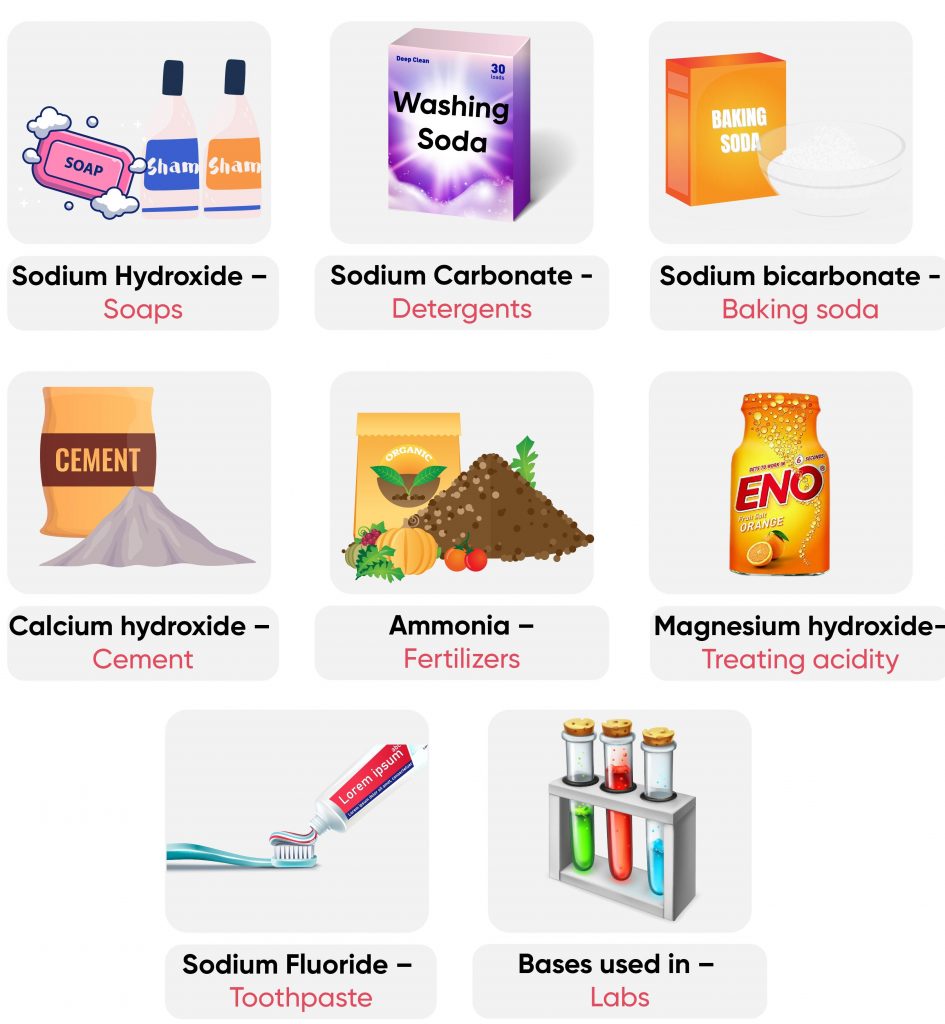
1. Calcium Hydroxide
- Found in- Lime water
- Explanation- Calcium hydroxide is present in lime water and is used in various applications such as water treatment and in the preparation of certain food products.
2. Ammonium Hydroxide
- Found in- Window cleaner
- Explanation- Ammonium hydroxide is commonly used in cleaning products. It helps in removing dirt and stains from surfaces like glass.
3. Sodium Hydroxide and Potassium Hydroxide
- Found in- Soap
- Explanation- These bases are strong and commonly used in the manufacture of soaps and detergents to help remove dirt and oils.
4. Magnesium Hydroxide
- Found in- Milk of magnesia
- Explanation- Magnesium hydroxide is used as an antacid to neutralize stomach acid and relieve indigestion and heartburn.
Natural Indicators of Acid and Bases
Natural indicators are substances that change colour when exposed to acidic or basic solutions. They are an essential tool in chemistry for identifying the nature of various substances. This section delves into some commonly used natural indicators and their colour-changing properties.
Types of Natural Indicators-
- Litmus Paper- Litmus paper is a popular indicator used to test whether a solution is acidic or basic. Blue litmus paper turns red in acidic solutions and red litmus paper turns blue in basic solutions, providing a clear and quick way to determine the nature of a substance.
- Turmeric- A common spice found in kitchens, turmeric changes colour in the presence of a base. It turns from yellow to red when it comes into contact with a basic substance, making it a handy and accessible indicator.
- China Rose Petals- The petals of the China rose can also be used as a natural indicator. They produce different colours in acidic and basic environments, shifting from pink in acidic solutions to green in basic solutions.
Also Check – Indicators for Acid and Base- A Guide for Students
Simple Experiments-
- Litmus Paper Test- Dip blue and red litmus papers in various household liquids like lemon juice (acidic) and soapy water (basic) to observe the colour changes.
- Turmeric Test- Mix a small amount of turmeric powder with water to form a paste. Apply this paste on a white paper and let it dry. Once dry, drop small amounts of lemon juice and soapy water on different parts of the paper to see the colour change.
- China Rose Petal Indicator- Crush China rose petals and soak them in water to extract the colour. Divide the solution into two parts. Add vinegar to one part (acidic) and baking soda solution to the other (basic) to observe the colour changes.
Class 7 – Chapter 4- Acids, Bases, and Salts- An Overview of Activities
Neutralisation Reaction
What is Neutralisation?
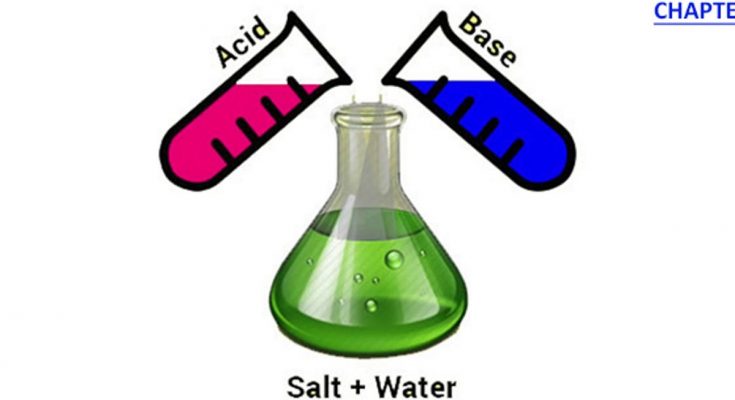
Neutralisation is a fundamental chemical reaction where an acid and a base react with each other to form salt and water. This reaction is significant in various practical applications and is a key concept in understanding the balance between acidic and basic substances.
The Reaction Process
The process of neutralisation can be simplified as follows- when an acid (which releases hydrogen ions in solution) and a base (which releases hydroxide ions) come into contact, they react to form water (H₂O) and an ionic compound called a salt. This reaction effectively neutralises the acidic and basic properties of the reactants.
Also Check – What is Neutralisation? – The Basics Explained
General Equation-
Acid+Base→Salt+Water
Acid+Base→Salt+Water (Heat is evolved during the process)
Practical Examples of Neutralization-
- Treating Bee Stings with Baking Soda- Bee venom is acidic. When a person is stung by a bee, applying a paste of baking soda (a basic substance) on the sting site can help neutralise the venom’s acidic effect, providing relief from pain and swelling.
- Treating Indigestion with Antacids- Indigestion often involves excessive stomach acid. Antacids, which are basic substances, are used to neutralise this excess acid, thereby alleviating discomfort and symptoms of acid reflux or heartburn
Example of Neutralization Reaction –
Hydrochloric acid (HCl)+Sodium hydroxide (NaOH)→Sodium chloride (NaCl)+Water (H2O)
Neutralisation in Everyday Life
Neutralisation is a process where acids and bases interact to nullify each other’s effects, resulting in the formation of water and salt.
1. Indigestion
- What happens? The stomach naturally contains hydrochloric acid (HCl) to help in digesting food. Sometimes, there’s too much acid, causing discomfort known as indigestion.
- How is it resolved? An antacid, like milk of magnesia, which has a basic substance called magnesium hydroxide (Mg(OH)2), is consumed. This antacid helps in neutralising the excess acid in the stomach.
- Explanation- When magnesium hydroxide reacts with hydrochloric acid, it forms water and magnesium chloride (a salt), reducing acidity and relieving indigestion pains.
2. Ant Bites
- What happens? When an ant bites, it releases formic acid into the skin, which causes irritation and pain.
- How is it resolved? By applying a basic solution like baking soda (sodium hydrogen carbonate) or calamine (which contains zinc carbonate), the acid from the ant bite is neutralised.
- Explanation- The base in baking soda or calamine interacts with the formic acid, neutralising the irritation caused by the ant bite.
Also Check – What is a Neutralisation Reaction?- Explained
3. Soil Treatment
- What happens? Continuous use of chemical fertilisers can make soil too acidic or too basic, which is not suitable for plants.
- How is it resolved?
- If soil is acidic- Quicklime or slaked lime are added. These are basic and help neutralise the soil’s acidity.
- If soil is basic- Organic matter, which is slightly acidic, is added to reduce the basic nature of the soil.
- Explanation- Adjusting the pH of the soil through these substances ensures a conducive environment for plant growth, as it neutralises excessive acidity or basicity.
4. Factory Wastes
- What happens? Factories often release waste containing acidic substances. If directly released into water bodies, these can harm aquatic life.
- How is it resolved? Basic substances are added to the acidic wastes, neutralising them before they are released into the environment.
- Explanation- This neutralisation process safeguards aquatic organisms by preventing the release of harmful acidic wastes into water bodies.
Types of Salts and Their Uses
Formation and Variety of Salts- Salts are versatile compounds formed through the neutralisation reaction between an acid and a base. These compounds have diverse structures and properties, leading to a wide range of uses in various fields.
Formation of salt through Neutralization
During the neutralisation process, the hydrogen ions from the acid and the hydroxide ions from the base combine to form water, while the remaining ions from both reactants form the salt. This reaction is fundamental in producing various types of salts.
Examples of Salts and Their Uses-
- Sodium Chloride (NaCl)- Commonly known as table salt, it is essential in cooking and food preservation. Sodium chloride enhances flavour and is used as a seasoning and preservative in a wide array of culinary applications. It is also used in de-icing roads and in water softening.
- Baking Soda (Sodium Bicarbonate)- A base itself, baking soda is used in cooking, particularly in baking, as it reacts with acidic components in the batter to release carbon dioxide, causing the batter to rise. It’s also a popular cleaning agent due to its mild abrasive properties and is used in personal care products like toothpaste.
Salt Uses in Various Fields-
- In Cooking- Beyond flavour enhancement, salts play a role in food preservation, such as in curing meats and pickling vegetables, due to their ability to inhibit the growth of bacteria.
- In Cleaning- Certain salts are used in household cleaners for their ability to react with and break down stains, grease, and dirt.
- In Agriculture- Salts are used in fertilisers to provide essential nutrients to plants. For example, potassium chloride is a common ingredient in fertilisers.
Acid Rain- Its Causes and Effects
The Phenomenon of Acid Rain- Acid rain is a significant environmental concern, resulting from chemical reactions in the atmosphere. It occurs when pollutants like sulphur dioxide and nitrogen oxides, often released by burning fossil fuels, combine with atmospheric moisture.
- Formation- Sulphur dioxide and nitrogen oxides react with water, oxygen, and other chemicals in the atmosphere to form sulfuric and nitric acids. These acids then mix with rain, resulting in acid rain.
- Environmental Impact- Acid rain has detrimental effects on natural ecosystems. It can acidify lakes and streams, making them uninhabitable for many aquatic species. It also depletes essential nutrients in the soil, impacting plant growth and forest ecosystems.
- Effect on Buildings and Monuments- Beyond natural environments, acid rain also poses a threat to buildings and historical monuments, especially those made of limestone and marble, as the acidic components erode these materials over time.
Acid-Base Reactions in Everyday Life
Everyday Chemistry- The interactions between acids and bases are not just confined to the laboratory; they are integral to many daily processes and products.
- Digestive Process- Our stomach uses hydrochloric acid for digestion, breaking down food into absorbable nutrients. This acid activates enzymes necessary for protein digestion.
- Antacids in Action- To counteract excess stomach acid, causing discomfort like heartburn or indigestion, antacids, which are basic substances, are used. They neutralise the stomach acid, providing relief.
- Common Household Examples- Various household substances involve acid-base reactions. For instance, the carbonation in sodas is due to carbonic acid, while cleaning agents often contain bases that neutralise and dissolve stains and grease.
Safety Precautions
Ensuring Safe Handling of Acids and Bases- Working with acids and bases, whether in a laboratory setting or at home, demands adherence to safety protocols. Proper handling and disposal are essential to prevent accidents and ensure safety.
Handling in the Laboratory and at Home-
- Use of Appropriate Containers- Always store acids and bases in appropriate containers that are clearly labelled to avoid confusion and accidental misuse.
- Avoid Direct Contact- Direct contact with skin or eyes can be harmful. It’s crucial to handle these substances carefully to prevent splashes or spills.
- Ventilation- Ensure good ventilation when handling volatile acids or bases to avoid inhaling fumes that can be harmful.
Also Check – 10 Facts About Acids and Bases
Importance of Wearing Protective Gear-
- Protective Eyewear- Always wear safety goggles to protect your eyes from splashes.
- Gloves and Lab Coat- Use gloves and a lab coat or apron to protect your skin and clothing from accidental spills.
- Face Shield- In situations where there is a risk of splashing or when handling particularly hazardous materials, a face shield should be worn.
Proper Disposal-
- Neutralisation- Before disposal, neutralise acids and bases. This can be done using a neutralising agent or by dilution with water, following the specific guidelines for the substance.
- Follow Local Regulations- Dispose of the neutralised substances according to local waste disposal regulations. Never pour them down the drain without ensuring they are neutralised and permissible to dispose of in this manner.
Curious Minds- Questions and Answers on Acids, Bases, and Salts
Why don’t all acids and bases completely dissolve in water?
- Not all acids and bases dissolve completely in water because of their different strengths. Strong acids and bases dissociate completely, while weak acids and bases do not. This is due to the different structures and bonding within their molecules.
Can acids and bases be found in a gaseous state?
- Yes, acids and bases can exist in gaseous forms. For example, hydrogen chloride gas (HCl) is a gaseous acid. When it dissolves in water, it forms hydrochloric acid.
Why does neutralisation not always result in a neutral pH of 7?
- Neutralisation results in a salt and water, but the pH of the resulting solution depends on the strengths of the acid and base involved. If a strong acid reacts with a weak base, the solution might still be slightly acidic, and vice versa.
How do antacids work to relieve heartburn?
- Antacids contain basic substances that neutralise the excess stomach acid causing heartburn, thereby relieving the discomfort. They react with the hydrochloric acid in the stomach to form salt and water.
Why is rain naturally slightly acidic?
- Rain is naturally acidic because carbon dioxide in the air dissolves in rainwater, forming a weak carbonic acid. This is a natural phenomenon, separate from the much stronger and harmful acid rain caused by pollutants.
What makes some salts edible while others are not?
- The safety of salts for consumption depends on their chemical components. Edible salts, like sodium chloride, are safe and necessary for human health. Other salts may contain toxic elements or have harmful properties making them unsafe for consumption.
Why does baking soda fizz when mixed with vinegar?
- Baking soda (sodium bicarbonate) reacts with the acetic acid in vinegar to produce carbon dioxide gas, water, and sodium acetate. The fizzing is due to the rapid release of carbon dioxide gas.
How does soap clean dirt and grease off surfaces?
- Soap is a base that reacts with the fatty acids in dirt and grease, forming soap scum that can be washed away with water. This reaction also helps to break down oils and fats, making it easier to remove them from surfaces.
Can the pH of a solution change over time?
- Yes, the pH of a solution can change over time due to reactions with substances in the air, like carbon dioxide, or due to the breakdown of some of the solution’s components.
Why is it dangerous to mix different household cleaners?
- Mixing different household cleaners can be dangerous because they may contain various acids and bases that can react violently with each other, producing toxic gases or causing explosions.
Also Check – Class 7- Chapter 4 – Acids, Bases and Salts – 4 Worksheets Solved and Unsolved
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (Long)
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (Short)
Also Check – NCERT Solutions For Class 7 Science Chapter 5 – Acids, Bases and Salts
Also Check – Acid Rain – Class 7 -Chapter 5-Acids, Bases and Salts
Also Check – Class 7 science -Chapter 5-Acids, Bases and Salts – Definition and Explanation of Important Keywords
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (Fill in the Blanks)
Also Check – Chapter 5- Acids, Bases and Salts-Class 7 Question and Answer (MCQ)