Table of Contents
What Are Acid-Base Indicators?
Acid-base indicators are substances that change colour when added to acidic or basic solutions. They are a key tool in chemistry for identifying the acidic or basic nature of materials. By observing the colour change, one can determine whether a substance is an acid, base, or neutral.
The Importance of Understanding Acids and Bases
Acids and bases are everywhere – from the food we eat to the cleaning agents we use, and even inside our bodies. Understanding their nature is crucial in fields like medicine, environmental science, and manufacturing. Indicators help us safely and efficiently determine the pH of substances, guiding our use and understanding of various materials in everyday life.
How Indicators Work
What is pH and Its Scale
pH is a scale used to specify how acidic or basic a water-based solution is. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, and those with a pH greater than 7 are basic. Understanding pH is crucial for gauging the chemical nature of substances in various applications.
Indicators and Their Color Change Mechanism
Indicators are typically weak acids or bases that exhibit a distinct colour change in different pH environments. This change occurs due to the alteration in the concentration of hydrogen (H⁺) or hydroxide (OH⁻) ions in a solution. For instance, litmus, a common indicator, turns red in acidic solutions (pH < 7) and blue in basic solutions (pH > 7).
Chemical Basis of the Colour Change
At a molecular level, the colour change happens because of the shifting equilibrium between the acid and base forms of the indicator. For a weak acid indicator, this equilibrium can be represented as-
HIn(aq) + H2O(l) ↔ In⁻(aq) + H3O⁺(aq)
In this reaction-
- HIn(aq) represents the acid form of the indicator in aqueous solution.
- H2O(l) is water.
- In⁻(aq) is the base form of the indicator in aqueous solution.
- H3O⁺(aq) is the hydronium ion in aqueous solution.
The ↔ symbol indicates that the reaction is in equilibrium, meaning it can proceed in both directions. This equilibrium shifts based on the pH of the solution, leading to a visible colour change in the indicator.
Also Check – A Young Explorer’s Guide to Acids and Bases
Common Acid-Base Indicators and Natural Indicators Around Us
In the realm of chemistry, indicators are crucial for determining the nature of solutions. This section combines information on common synthetic indicators like litmus paper and phenolphthalein with natural indicators such as turmeric and red cabbage juice.
Synthetic Indicators- Litmus Paper and Phenolphthalein
- Litmus Paper– Available in red and blue, litmus paper is a simple way to test for acidity or alkalinity. Blue litmus turns red in acidic conditions (pH < 7), while red litmus turns blue in basic conditions (pH > 7).
- Phenolphthalein– Used in titrations, phenolphthalein is colourless in acidic solutions and turns pink in basic solutions (pH above 8.2), making it a clear indicator of pH changes.
Natural Indicators- Turmeric and Red Cabbage Juice
- Turmeric– Contains curcumin, which changes to a reddish-brown colour in the presence of a base. A simple experiment involves applying turmeric paste on paper and adding a basic substance like soap to observe this change.
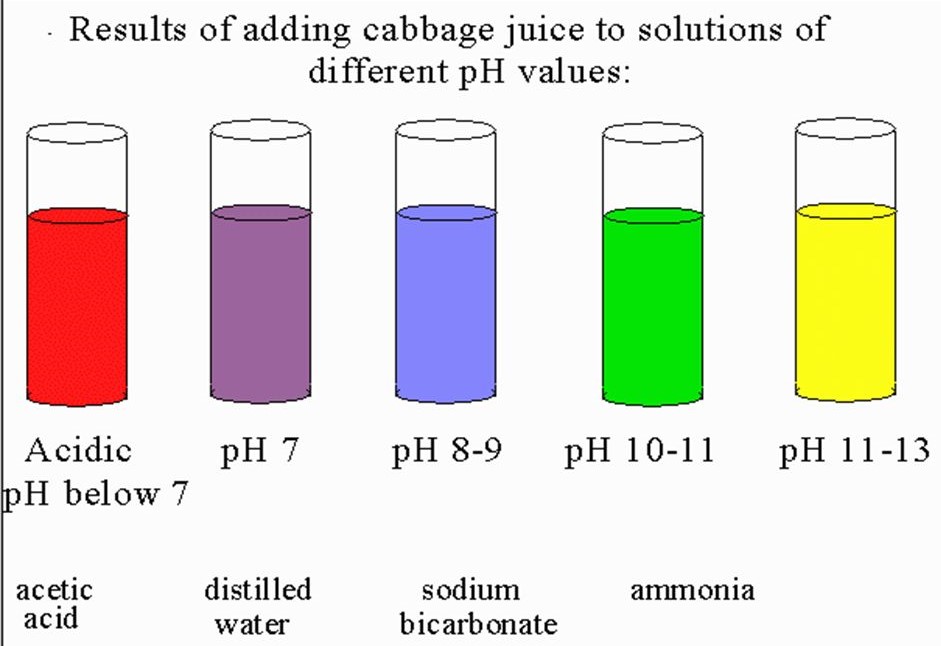
- Red Cabbage Juice- Acts as a natural pH indicator, changing from reddish in acidic solutions to greenish-blue in basic solutions. Boiling red cabbage to extract the juice makes for a versatile and safe indicator.
Also Check – Activity – China Rose as an Indicator
Chemical Structures and Color Changes of Phenolphthalein and Curcumin
In exploring acid-base indicators, understanding the molecular basis for their colour changes offers deep insights. Here we delve into the chemical structures of phenolphthalein and curcumin, explaining how their structural changes in different pH environments lead to observable colour changes.
Phenolphthalein in Acidic and Basic Conditions
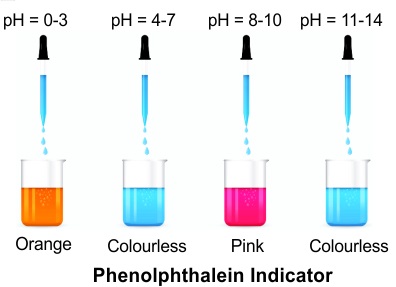
- Acidic Conditions- In an acidic environment, phenolphthalein’s structure does not absorb visible light, remaining colourless. This is due to the intact hydrogen ion attached to the molecule, keeping it in a form that does not interact significantly with visible light wavelengths.
- Basic Conditions- Under basic conditions, phenolphthalein undergoes a significant structural transformation. The loss of a hydrogen ion (deprotonation) leads to the formation of a conjugated system, which can absorb certain wavelengths of light. This absorption results in the reflection of pink or magenta hues, a clear indicator of a basic pH. The molecule now becomes a system with extended pi-electron delocalization, allowing it to absorb and reflect light differently than in its acidic form.
Curcumin’s Colour Transformation
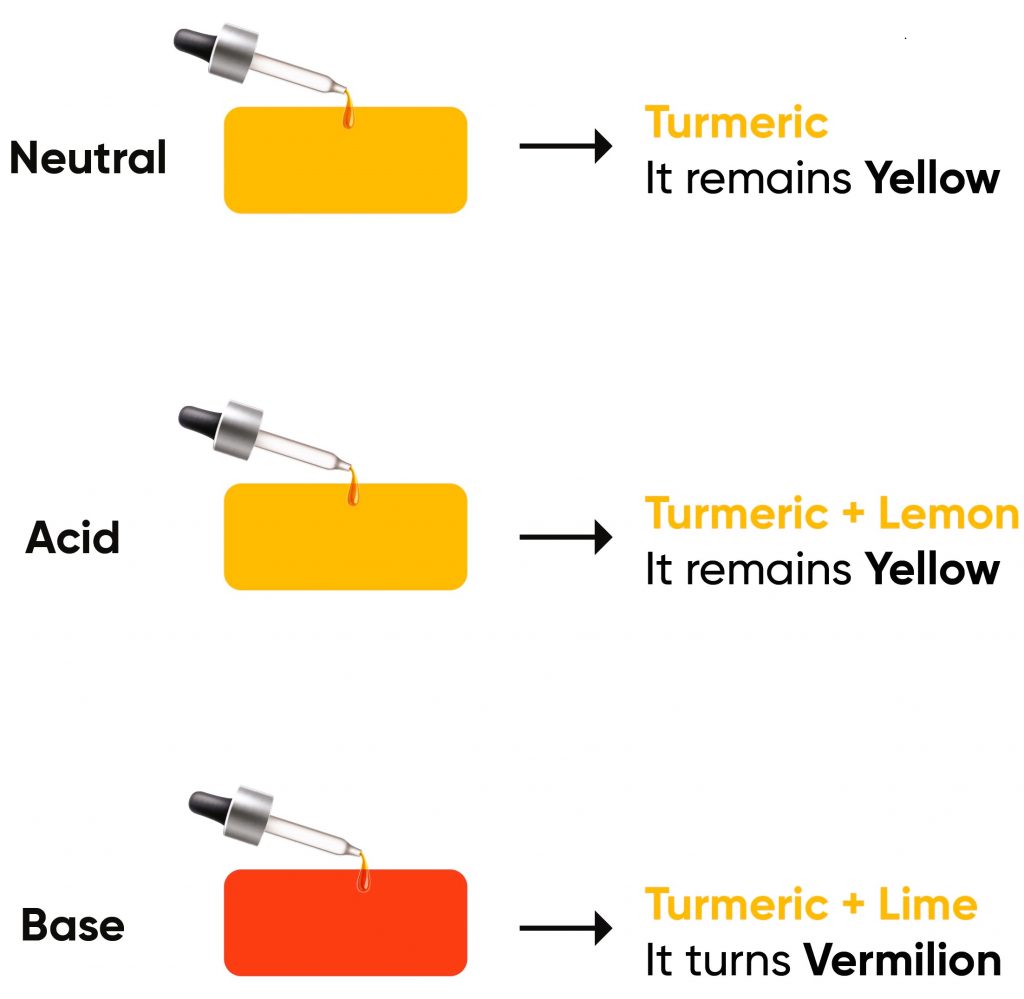
- Neutral/Acidic Conditions- Curcumin, the active component in turmeric, maintains a yellow colour in neutral and acidic settings. Its molecular structure, featuring a stable conjugated double bond system, does not undergo significant light absorption shifts in this pH range, thus retaining its yellow appearance.
- Basic Conditions- In a basic medium, curcumin experiences keto-enol tautomerism. This change is a rearrangement of chemical bonds within the molecule, specifically involving the transfer of a hydrogen atom and a shift in double bond positions. The new structure extends the conjugation of the double bonds, altering the light absorption spectrum of the molecule and resulting in a reddish-brown colour. This transformation is a direct result of the interaction with hydroxide ions in the basic medium, which facilitates the structural change.
Also Check – What is Neutralisation? – The Basics Explained
Universal Indicator and pH Paper
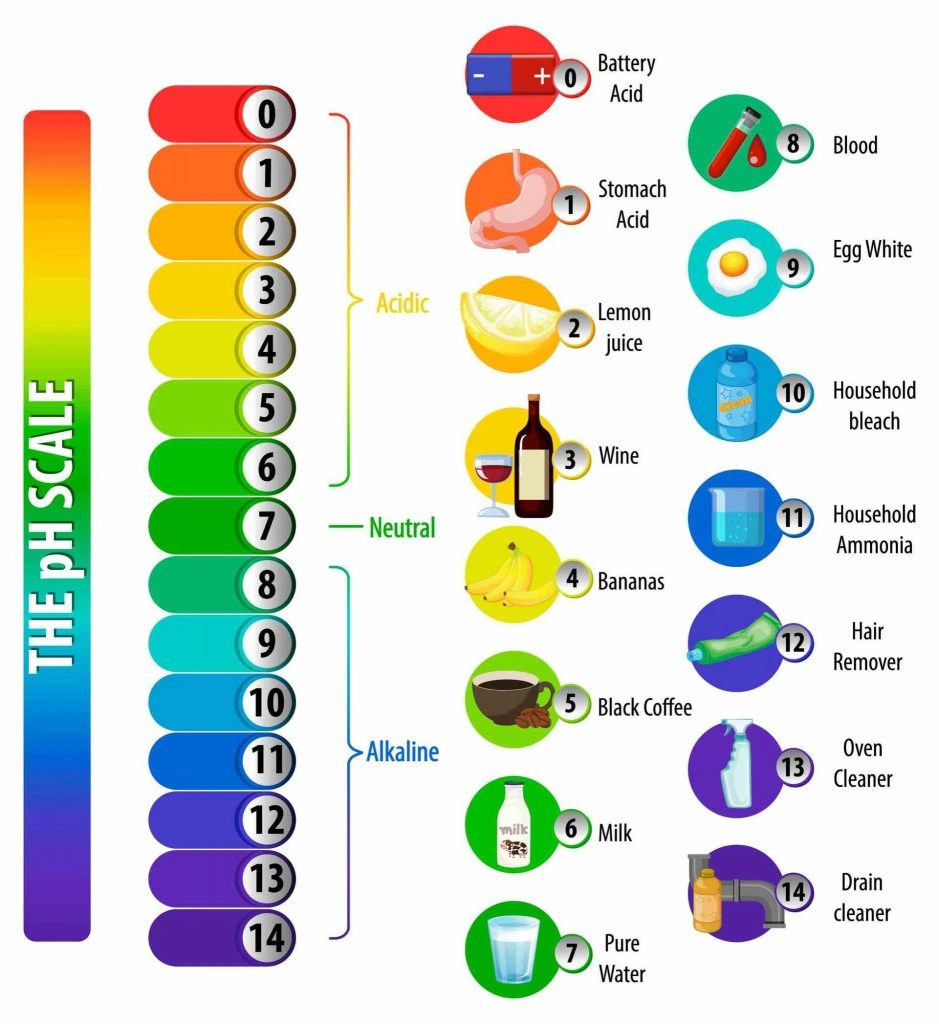
Understanding Universal Indicator
A universal indicator is a sophisticated blend of several different indicators. Unlike single indicators like litmus or phenolphthalein, a universal indicator can provide a broad spectrum of colours, each corresponding to a specific pH level. This allows it to give a more comprehensive view of a solution’s acidity or basicity.
- Colour Range- The colour spectrum of a universal indicator ranges from red, indicating a highly acidic solution (pH around 1), through greens, indicating neutral (pH 7), to dark purple or blue for highly alkaline solutions (pH around 14).
- Application- A few drops of universal indicator added to a solution can quickly show its pH, making it an invaluable tool in labs for determining the pH of a wide range of substances.
pH Paper
pH paper is another tool used to determine the approximate pH of a solution. It is a special paper that has been treated with a universal indicator.
- Usage- When a strip of pH paper is dipped into a solution, it changes colour based on the solution’s pH level. The colour can then be compared to a colour chart that accompanies the pH paper, giving an approximate pH value.
- Advantages- pH paper is particularly useful for quick, approximate readings. It is easy to use, making it a popular choice in educational settings and for rapid field testing where precise measurements are not necessary.
Choosing the Right Indicator
Selecting an Appropriate Indicator
Choosing the right indicator for an acid-base reaction is crucial for accurate pH measurement. The selection largely depends on the pH range of interest and the specific application.
- Understanding pH Range- Different indicators change colour at different pH levels. For instance, litmus is suitable for determining whether a solution is broadly acidic or basic, but it cannot provide precise pH values. On the other hand, pH paper or a universal indicator offers a broader range and can pinpoint the pH value more accurately.
- Specific Applications- The choice of indicator also depends on the specific application. For laboratory titrations, where precise pH readings are necessary, indicators like phenolphthalein, which has a clear and distinct colour change at a specific pH, are preferred. In contrast, for general classroom demonstrations or simple tests, litmus or red cabbage juice might suffice.
Why Certain Indicators Suit Specific Reactions
- Indicator Sensitivity- Some indicators are more sensitive to pH changes than others. For example, phenolphthalein is suitable for reactions that occur near a neutral pH, as it changes colour around a pH of 8.2.
- Chemical Compatibility- The chemical nature of the solution can also influence the choice of indicator. Some indicators might not work effectively in certain chemical environments due to interactions with other substances present in the solution.
- Visibility of Colour Change- The clarity and visibility of the colour change are also important. Some indicators might produce a very subtle colour change, which can be difficult to observe, especially in a classroom setting.
Safety Precautions and Tips
Prioritising Safety in Acid-Base Experiments
When handling acids, bases, and indicators, especially in a laboratory or during experiments, it’s crucial to follow safety guidelines to prevent accidents and ensure a safe learning environment.
- Wear Proper Protective Equipment- Always use safety goggles, gloves, and a lab coat. This protective gear guards against splashes or accidental contact with chemicals.
- Handle Chemicals with Care- Acids and bases should be handled with caution. Pour them slowly to avoid splashes, and never mix them abruptly, as this could lead to violent reactions.
- Ensure Good Ventilation- Work in a well-ventilated area to avoid inhaling fumes, especially when dealing with volatile substances.
- Use Correct Containers and Labels- Store all chemicals, including indicators, in appropriate containers with clear labels. This prevents confusion and accidental misuse.
- Know Emergency Procedures- Be familiar with the emergency protocols, including the location of eyewash stations and first aid kits. In case of spills or contact with skin, immediate action is vital.
- Safe Disposal of Chemicals- Dispose of chemicals and used indicators according to established guidelines. Certain chemicals require neutralisation or specific disposal methods to prevent environmental damage.