Activity 4.3, featured in the chapter “Acids, Bases, and Salts,” is an educational and engaging experiment that introduces the concept of natural indicators in chemistry, specifically using China rose petals. This activity aims to demonstrate how China rose can be used to determine the acidic or basic nature of various substances.
Class 7 – Chapter 4- Acids, Bases, and Salts- An Overview of Activities
Activity 4.3- China Rose as an Indicator
Materials Needed
- China rose (Gudhal) petals
- Water
- Various test solutions (like lemon juice, orange juice, vinegar, milk of magnesia, baking soda, lime water, sugar, common salt)
- Containers (beakers or cups)
Preparation of China Rose Indicator-
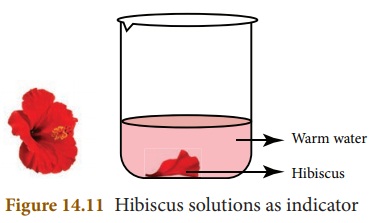
- Materials Used- China rose (Gudhal) petals, warm water, beaker.
- Procedure-
- Place China rose petals in a beaker.
- Add warm water and allow the mixture to sit until the water becomes coloured.
Testing Procedure-
- Objective- To observe the effect of the China rose indicator on various solutions.
- Method- Add five drops of the indicator to each test solution and observe the change in colour.
Observation and Interpretation-
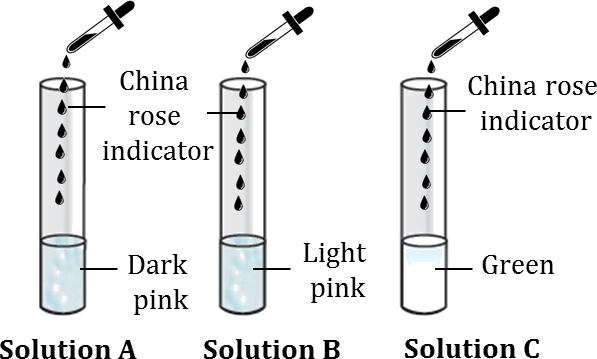
- Acidic Solutions- Turn dark pink (magenta).
- Basic Solutions- Turn green.
- Neutral Solutions- May not show significant colour change.
Table of Observations with Expected Results-
| S. No. | Test Solution | Initial Colour | Final Colour | Nature of Solution |
|---|---|---|---|---|
| 1 | Shampoo (dilute solution) | Varies based on shampoo | Likely Green | Likely Basic |
| 2 | Lemon Juice | Yellow to Greenish-yellow | Dark Pink/Magenta | Acidic |
| 3 | Soda Water | Colourless | Likely Green | Likely Basic |
| 4 | Sodium Hydrogen Carbonate Solution | Colourless | Green | Basic |
| 5 | Vinegar | Colourless to pale yellow | Dark Pink/Magenta | Acidic |
| 6 | Sugar Solution | Colourless | No Significant Change/Likely remains Colourless | Likely Neutral |
| 7 | Common Salt Solution | Colourless | No Significant Change/Likely remains Colourless | Likely Neutral |
Remarks-
- Shampoo- Most shampoos are slightly basic to maintain the natural pH of the hair and scalp.
- Lemon Juice- It’s acidic due to the presence of citric acid.
- Soda Water- Contains carbonic acid and bicarbonate ions, but due to the bicarbonate ions, it usually acts as a base.
- Sodium Hydrogen Carbonate Solution- Basic in nature.
- Vinegar- Contains acetic acid, hence it is acidic.
- Sugar Solution- Sugar is neutral, and thus, the indicator colour likely won’t change.
- Common Salt Solution- Salt solution is neutral in nature.
The final colours mentioned are based on theoretical knowledge and general chemical principles. For precise results, conducting an actual experiment is advised.
Also Check – Indicators for Acid and Base- A Guide for Students
Conceptual Understanding
- Natural Indicators-
- China rose is a natural indicator that changes colour when added to different types of solutions. This colour change is a visual representation of the solution’s pH level.
- Acidic and Basic Solutions-
- Acidic solutions cause the China rose indicator to turn dark pink or magenta. Basic solutions, on the other hand, change the indicator to green.
- Neutral Solutions-
- Neutral solutions, such as sugar or common salt solutions, will not significantly alter the colour of the China rose indicator.
- Chemical Reaction and pH Sensitivity-
- The colour change is a result of the chemical reaction between the China rose pigments and the substances in the solution, reflecting their pH level. The pH scale measures how acidic or basic a substance is.
- Real-life Application-
- This activity connects the theoretical concept of acids and bases to real-life scenarios, showing how natural substances can indicate the nature of household items.
- Observational Skills-
- Students are encouraged to keenly observe the colour changes, enhancing their observational and analytical skills in scientific experiments.
Learning Outcomes
- Identification of Acids and Bases- Students learn to identify substances as acidic or basic using a natural indicator.
- Understanding pH- The activity introduces the concept of pH in a practical, visually engaging way.
- Natural Science Application- Demonstrates the use of natural materials in scientific investigations, fostering an appreciation for nature’s role in science.
- Critical Thinking and Analysis- Encourages students to think critically and analyse their observations in a structured scientific manner.
Conclusion
Activity 4.3 provides a hands-on learning experience about acids, bases, and neutral solutions using a natural indicator. By utilising China rose petals, students can visually understand the reaction of natural pigments with various substances, leading to a deeper appreciation of the role of nature in scientific discovery. This activity not only enriches their understanding of chemical properties but also enhances their observational and analytical skills, essential for scientific studies.
Also Check- Young Explorer’s Guide to Acids and Bases
Also Chekc – Rapid Revision – Class 7 Science – Chapter 5-Acids, Bases and Salts
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 5- Acids, Bases and Salts
Also Check – NCERT Solutions For Class 7 Science Chapter 5 – Acids, Bases and Salts
Also Check – Class 7- Chapter 4 – Acids, Bases and Salts – 4 Worksheets Solved and Unsolved
Class 7 science -Chapter 5-Acids, Bases and Salts – Definition and Explanation of Important Keywords
Also Check -Class 7 science -Chapter 5-Acids, Bases and Salts- Complete Notes
