Welcome to a special part of science – the world of acids and bases! the world of acids and bases. These are more than just words from your science book; they’re a part of everything around us – in the kitchen, the garden, and even in our own bodies!
Have you ever tasted lemon juice and found it sour? That sourness comes from something called an acid. Acids are found in lots of things like oranges, vinegar, and even in raindrops. Bases are different – they’re usually not sour but bitter, and they feel kind of slippery, like the soap you use when you wash your hands.
What Happens When Acids and Bases Meet?
Something really cool happens when an acid and a base mix together – they can make new things like salt and water. It’s like they’re having a little party and creating something new to surprise us!
A Journey of Discovery!
We’re going to find out so many amazing things about acids and bases. From how they behave to some awesome experiments you can try at home. So, let’s start this adventure and discover the amazing secrets of acids and bases together!
Table of Contents
What Exactly Are Acids and Bases?
Acids: More Than Just Sour
Sure, we learned that acids taste sour, like the lemon juice in your drink. But acids are not just about the taste; they’re quite interesting! Some acids, like the one in vinegar, are mild and used in cooking. And did you know, rain contains a kind of acid too? But don’t worry, it’s very weak and not harmful like the acids in a laboratory.
Bases: Beyond the Bitterness
Bases, as we found out, can be bitter and slippery. But they’re also super useful! Take baking soda, a common base found in our kitchens. It’s used to make our cakes and cookies rise and become fluffy. Bases are not just in our food; they help in many everyday tasks.
Litmus Test for Acids and Bases
Remember the fascinating litmus paper? It’s a cool way to tell if something is an acid or a base. When an acid comes in contact with blue litmus paper, the paper turns red. And when a base meets red litmus paper, the paper turns blue. This simple test is a fun way to explore the nature of acids and bases.
Everyday Examples of Acids and Bases
Now that we know a bit more about acids and bases, let’s find out where we can spot them in our daily lives. You might be surprised to see how common they are!
Acids Around Us
Apart from the citric acid in lemons and vinegar in our kitchens, there are other acids we use often. Have you ever enjoyed a fizzy soda? That fizziness comes from an acid too, called carbonic acid. Also, yoghurt contains lactic acid, which gives it a slightly sour taste. These acids are safe and add flavour to our food.
Bases in Our Homes

Bases are not left behind in our daily routine. Do you like how clean and shiny your clothes are after washing? That’s thanks to laundry detergents, which are bases. Another example is toothpaste, which is a base too, helping keep our teeth clean and healthy. Like acids, bases in our homes are generally safe and very useful.
Acids and Bases in Cooking
In cooking, acids and bases play a special role. Lemon juice (an acid) is used in marinades to tenderise meat, and baking soda (a base) helps in baking delicious cakes. They are like the secret ingredients that make our food taste great and look wonderful!
Acids and bases are not just in science labs; they are a part of our everyday life, making things tastier, cleaner, and more fun. Isn’t it fascinating how science is involved in so many parts of our day?
Basic Theories of Acids and Bases
In our adventure through the world of acids and bases, it’s time to understand a bit about the science behind them. There are three main theories that scientists use to explain what acids and bases are. These theories might sound complex, but we’ll explore them in a fun and simple way!
Arrhenius Theory
The first theory is by a scientist named Arrhenius. He said that acids are substances that create H+ ions in a solution. Think of it like adding a tiny, invisible ingredient to water that makes it acidic. Bases, according to Arrhenius, are substances that produce OH– ions in a solution, which makes them the opposite of acids.
Bronsted-Lowry Theory
The next theory is from Bronsted and Lowry. They described acids as ‘proton donors.’ A proton is a tiny particle, even smaller than an H+ ion. So, in this theory, acids give away these tiny particles. Bases, on the other hand, are ‘proton acceptors,’ which means they take these tiny particles from acids.
Lewis Theory
The third theory comes from a scientist named Lewis. He had a different view and said that acids are substances that accept a pair of electrons, and bases are those that donate a pair of electrons. It’s like acids are asking for a tiny part of the atom, and bases are ready to give it.
Each of these theories helps scientists understand more about how acids and bases work. They might seem a bit tricky, but remember, it’s all about tiny particles moving around and changing how a substance behaves!
Also Check – What is a Neutralisation Reaction?- Explained
pH Scale: Understanding Acidity and Basicity
Now, let’s talk about something called the pH scale, which is a really cool tool scientists use to understand how acidic or basic a substance is.
What is the pH Scale?
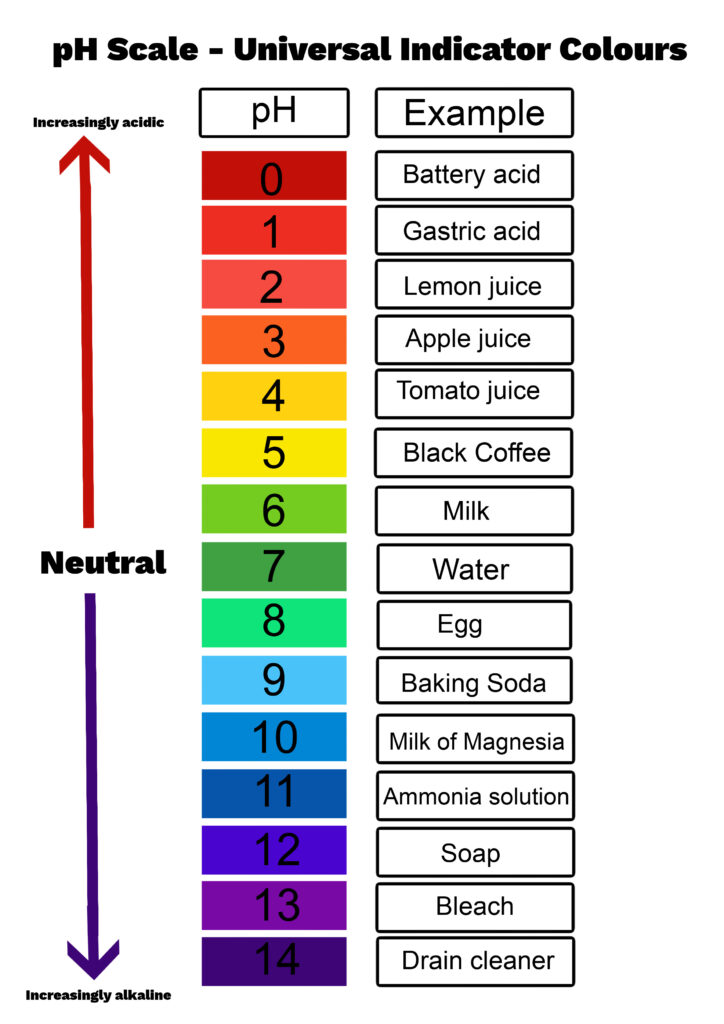
The term ‘pH’ stands for ‘potential of hydrogen.’ Imagine this scale as a ladder from 0 to 14. Each step on this ladder tells us how acidic or basic a substance is. The lower the number, the more acidic the substance is. So, a substance with a pH of 0 is super acidic. On the other hand, the higher the number, the more basic (or alkaline) the substance is, with 14 being the most basic.
Acids, Bases, and Neutrals on the pH Scale
Water, which is neutral, sits right in the middle of our pH ladder at 7. Anything below 7 is an acid, like lemon juice or vinegar. Anything above 7 is a base, like baking soda or soap.
The pH scale helps us to measure and compare the acidity or basicity of different substances. It’s like a secret code to know if something is an acid, a base, or just neutral like water.
Properties of Acids and Bases
Properties of Acids
- Taste Sour: You already know about the sour taste of acids like lemon juice and vinegar.
- React with Metals: Acids react with some metals to produce hydrogen gas. Have you seen bubbles when vinegar is poured on baking soda? That’s a reaction happening!
- Conduct Electricity: Acids can conduct electricity. This is because they have charged particles when dissolved in water.
- Change Litmus Paper Colour: Acids turn blue litmus paper red. It’s like a secret message being revealed!
- Corrosive Nature: Some strong acids can be corrosive, which means they can wear away some materials. That’s why we must be careful while handling them.
Properties of Bases
- Taste Bitter: Bases like baking soda have a bitter taste. But remember, not all bases should be tasted.
- Feel Slippery: Bases feel slippery, like soap. That’s why soap helps clean things so well!
- Conduct Electricity: Just like acids, bases can also conduct electricity when dissolved in water.
- Change Litmus Paper Color: Bases turn red litmus paper blue. It’s their way of saying “I’m a base!”
- Soapy Texture: When you touch bases, they often feel soapy. This texture is unique to them.
Also Check – What is Neutralisation? – The Basics Explained
Fun Experiments and Activities with Acids and Bases
Now, it’s time for some exciting experiments and activities! Remember to always have an adult with you for safety while you do these fun science experiments.
Experiment with Litmus Paper

You’ve learned how acids and bases can change the colour of litmus paper. Let’s try this out!
- Materials Needed: Red and blue litmus paper, lemon juice, baking soda, vinegar, soap solution, and water.
- What to Do: Dip blue litmus paper in lemon juice and watch it turn red. Then, dip red litmus paper in a soap solution and see it turn blue. You can try with other substances like vinegar (acidic) and a baking soda solution (basic) to see what happens!
- What We Learn: This experiment helps us understand how acids and bases react differently with litmus paper.
Baking Soda and Vinegar Volcano

This classic experiment is a fun way to see an acid-base reaction.
- Materials Needed: A small bottle or container, baking soda, vinegar, dish soap, food colouring (optional), and a tray to catch the ‘lava.’
- What to Do: Put the bottle on the tray. Add a few tablespoons of baking soda, a squirt of dish soap, and food colouring inside the bottle. Then, pour vinegar into the bottle and watch the bubbly ‘lava’ eruption!
- What We Learn: This experiment shows how an acid (vinegar) reacts with a base (baking soda) to produce carbon dioxide gas, causing the bubbly eruption.
Colour Changing Milk
- Materials Needed: Milk (whole or 2%), food colouring (multiple colours), dish soap, cotton swabs, and a shallow dish.
- What to Do: Pour milk into the dish just enough to cover the bottom. Add drops of different food colours. Dip a cotton swab in dish soap and gently touch the milk’s surface with the soap-covered swab. Watch the colours dance and swirl!
- What We Learn: This shows how soap, acting as a base, changes the surface tension of milk, causing the colours to move in fascinating patterns.
Invisible Ink with Lemon Juice

- Materials Needed: Lemon juice, a small bowl, a cotton swab or paintbrush, white paper, and a heat source like a lamp or hairdryer.
- What to Do: Dip the swab or brush in lemon juice and write a secret message on the paper. Allow it to dry completely. Then, hold the paper near a heat source and watch your message appear!
- What We Learn: The acid in the lemon juice weakens the paper. When heated, the weakened parts of the paper brown faster, revealing the message.
Homemade pH Indicator
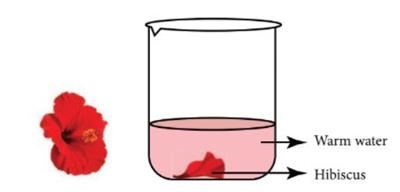
- Materials Needed: Red cabbage, boiling water, a blender, filter paper or coffee filter, clear cups or glasses, and various household liquids (like lemon juice, soda, baking soda solution, vinegar, soap water).
- What to Do: Blend red cabbage with boiling water to create a purple juice. Filter the mixture to get a clear solution. Pour some cabbage juice into each cup. Add different liquids to each cup and observe the colour change.
- What We Learn: Red cabbage juice acts as a natural pH indicator, changing colour in acidic and basic solutions, showing us the pH level of different substances.
Also Check – Indicators for Acid and Base- A Guide for Students
Curious Minds: Acids and Bases Q&A
Question: Why can’t we taste every acid and base to find out what they are?
Answer: Even though acids taste sour and bases taste bitter, it’s not safe to taste them all because some can be harmful. We should only taste things that are meant to be eaten, like foods that naturally have acids (like oranges) or bases (like baking soda in cookies).
Question: If I mix an acid and a base, will they always make salt and water?
Answer: Most of the time, when an acid and a base mix, they neutralise each other and form salt and water. But the type of salt might be different depending on the acid and base used.
Question: Can something be both an acid and a base?
Answer: Yes, some substances can act as both an acid and a base, depending on what they are mixed with. These are called ‘amphoteric’ substances. Water is a common example of this.
Question: Are acids and bases dangerous?
Answer: Some acids and bases can be dangerous, like the strong acids in car batteries or strong bases in some cleaning products. That’s why we must be careful and use them safely. But many acids and bases we use every day, like vinegar (an acid) or baking soda (a base), are safe when used correctly.
Question: How does the pH scale help us in real life?
Answer: The pH scale can help us understand the properties of different substances. For example, farmers use it to check soil health, and doctors use it to diagnose certain medical conditions.
Question: Why does litmus paper change colour with acids and bases?
Answer: Litmus paper has substances from lichens that change colour when they come into contact with acidic or basic substances. This change helps us identify whether the substance is an acid or a base.
Question: Can acids and bases be found in our bodies?
Answer: Yes, our stomach contains hydrochloric acid which helps in digestion, and our blood has a slightly basic pH to keep us healthy.
Question: Is rainwater an acid or a base?
Answer: Normal rainwater is slightly acidic because it mixes with carbon dioxide in the air to form carbonic acid. But it’s usually not harmful. However, rain can become more acidic due to pollution, which is called acid rain.
Question: What happens to the pH level of a lake if acid rain falls into it?
Answer: If acid rain falls into a lake, the pH level of the lake water can decrease, making it more acidic. This can affect the plants and animals living in the lake, as they might not be able to survive in more acidic conditions.
Question: Can acids and bases cancel each other’s effect?
Answer: Yes, when an acid and a base mix in the right proportions, they can neutralise each other. This means they can cancel out each other’s acidic or basic properties, often forming salt and water as a result.
Question: Are there any acids and bases in outer space?
Answer: Scientists believe that there are acids and bases in outer space. For example, ammonia, which is a base, has been found in the atmospheres of planets and moons. Scientists are studying these to learn more about space chemistry.
Question: Do all acids and bases have the same strength?
Answer: No, acids and bases can vary in strength. Some are very strong and can be dangerous, like sulfuric acid, while others are weak, like the acetic acid in vinegar.
Question: Why do we use baking soda to relieve heartburn?
Answer: Heartburn is caused by stomach acid. Baking soda is a base, and when you take it, it neutralises some of the stomach acid, relieving the heartburn feeling.
Question: Is the ocean acidic or basic?
Answer: The ocean is slightly basic, with a pH level around 8. However, the ocean’s pH is decreasing (becoming more acidic) due to absorbing carbon dioxide from the air, a concern for marine life.
Question: Can we see the pH change of a substance?
Answer: We can’t see the pH change just by looking, but we can use indicators like litmus paper or pH strips. Some natural substances, like red cabbage juice, can also show pH change by changing colours.
Question: Are there any edible bases like there are edible acids (like lemon juice)?
Answer: Yes, many edible substances are basic. For example, baking soda used in cooking is a base. It helps to balance the flavour and texture in recipes.
