Table of Contents
Chapter 4 – Heat -Notes – Explained in details
Heat is a fundamental concept in science that affects everything around us, from the weather to how we cook our food. This chapter introduces the idea of heat, its measurement, and how it’s transferred between objects. You’ll learn about the difference between hot and cold objects, how we measure temperature with thermometers, and the scales used for measurement. We explore different ways heat moves—through conduction in solids, convection in liquids and gases, and radiation without any medium. The chapter also touches on practical applications of heat, like in designing energy-efficient buildings and choosing appropriate clothing for different temperatures. By the end of this chapter, you’ll understand the basic principles of heat and how it impacts our daily lives.
Hot and Cold Objects
Our ability to feel temperature through touch is a basic yet crucial part of understanding the world around us. When we touch an object, we can often tell if it’s hot or cold. This section explains how we differentiate between hot and cold objects using our sense of touch and discusses the reliability and limitations of this method.
Using Touch to Determine Temperature
- Sense of Touch- Our skin is sensitive to temperature changes, allowing us to perceive whether an object is hot or cold.
- Immediate Perception- As soon as we touch something, we can usually tell its temperature. This helps us in everyday activities, like testing water temperature or feeling if food is cooked.
Reliability of Touch
- Subjective Nature- The sense of touch is not always reliable. What feels hot to one person might feel warm to another.
- Surrounding Environment- Our perception of temperature can be influenced by the environment. For instance, a metal object feels colder than a wooden one in the same room because metal conducts heat away from our hand faster.
Safety Precautions
- Risk of Burns- Hot objects can cause burns. It’s important to be cautious, especially with very hot items like cooking utensils or irons.
- Using Protection- When handling hot objects, use protective gear like oven mitts or tongs.
- Children’s Safety- Teach children to be cautious around hot objects and supervise them to prevent accidents.
Also Check – Class 7 science -Chapter 4 – Heat – Definition and Explanation of Important Keywords
Temperature and Thermometers
The concept of temperature is fundamental in understanding heat. This section elaborates on what temperature signifies, introduces thermometers as measurement tools, and explains the Celsius and Fahrenheit scales.
What is Temperature?
- Basic Definition- Temperature is a measure indicating how hot or cold an object is, directly related to the kinetic energy of the particles in the substance.
- Heat Energy Indicator- It reflects the amount of heat energy in an object. The higher the temperature, the more energetic the particles within the substance.
Thermometers- Precision in Measurement
- Role of Thermometers- They are devices specifically designed to measure temperature accurately and consistently.
- Types and Uses- There are various kinds of thermometers, such as digital, mercury, and alcohol, each suitable for different situations like medical assessments, scientific experiments, or everyday use.
Understanding Celsius and Fahrenheit Scales
- Celsius Scale– This scale, where water freezes at 0°C and boils at 100°C, is commonly used globally. It’s based on the properties of water and is crucial for scientific research, cooking, and weather reporting.
- Fahrenheit Scale- Primarily used in the United States, it sets the freezing point of water at 32°F and boiling point at 212°F. This scale is often used in daily weather forecasts and household appliances like ovens.
- Scale Conversion- Understanding how to convert temperatures from one scale to another.F = (9/5)C + 32″ for Fahrenheit and “C = (5/9)(F – 32)
Engaging in Temperature Activities
- Experiential Learning- Activities like measuring the temperature of various objects or environments help understand the abstract concept of temperature.
- Practical Demonstrations- Experiments that involve observing the effects of temperature changes on substances (e.g., melting ice, boiling water) provide tangible experiences with temperature concepts.
Clinical and Laboratory Thermometers
This section covers two specialised types of thermometers- clinical and laboratory thermometers. Each serves a different purpose, and understanding their usage and handling is crucial.
Clinical Thermometers
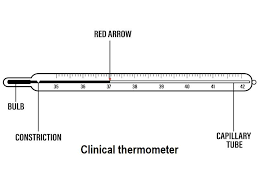
- Purpose- Clinical thermometers are specifically designed for measuring human body temperature. They are commonly used in medical settings and at home.
- Design- These thermometers are usually slender and have a constriction in the tube to hold the mercury or other temperature-sensitive liquid in place once removed from the body.
- Usage- To measure body temperature, the thermometer is placed under the tongue or in the armpit. After a few minutes, the temperature can be read from the scale.
- Reading- Careful reading is essential to get an accurate measurement. The temperature is usually measured in degrees Celsius or Fahrenheit.
Laboratory Thermometers
- Broader Range- Laboratory thermometers are designed to measure a wider range of temperatures compared to clinical thermometers.
- Applications- They are used for various scientific experiments in schools, colleges, and professional labs.
- Design and Usage- Unlike clinical thermometers, they don’t have a constriction in the tube and can measure both high and low temperatures. They are used by immersing or holding them in the substance whose temperature is to be measured.
Safe Handling and Reading
- Handling Precautions- Both types of thermometers should be handled with care to avoid breakage, as they can contain substances like mercury which are harmful.
- Reading Accuracy- Ensure the thermometer is clean and read at eye level to avoid parallax errors.
- After Use- It’s important to clean and disinfect clinical thermometers after use, especially when used by multiple people.
Also Check -Chapter 3 Heat Activities: Simple Experiments for Class 7
Heat Transfer
In this section, we delve into the concept of heat transfer, which is the process of heat moving from one object to another. Understanding how heat transfer works is crucial in both natural phenomena and everyday applications.
How Heat Moves
- Basic Principle- Heat always flows from a hotter object to a cooler one until both reach the same temperature.
- Transfer Methods- Heat can move in three primary ways- conduction, convection, and radiation. Each method has its unique characteristics and occurs in different circumstances.
The Concept of Heat Flow
- Energy Transfer- Heat flow is essentially the transfer of thermal energy between objects or within an object.
- Equilibrium- The ultimate goal of heat transfer is to reach thermal equilibrium, where no net heat flow occurs between objects in contact; they are at the same temperature.
Also Check – Heat facts for kids
Practical Implications
- Daily Life- We experience heat transfer daily, like when a spoon in a hot cup of tea becomes warm (conduction), or when we feel a warm breeze (convection).
- Technology and Industry- Heat transfer principles are critical in designing systems like heaters, refrigerators, and even buildings.
- Natural Processes- Understanding heat transfer helps explain natural phenomena like sea breezes, land breezes, and global weather patterns.
Also Check – NCERT Solutions For Class 7 Science Chapter 4 – Heat
Conduction
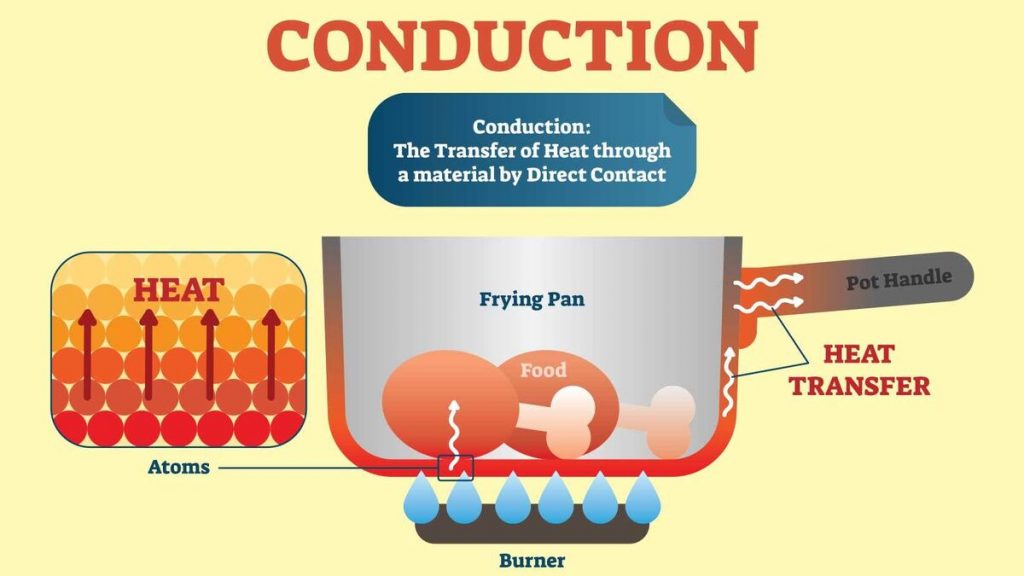
Conduction is a key method of heat transfer, particularly in solids. This section explores how conduction works, the role of different materials in conducting heat, and how to experiment with this concept.
Heat Transfer in Solids
Heat Transfer in Solids through Conduction
- Direct Contact Mechanism- In solids, conduction occurs when heat is transferred through direct contact between particles. The particles themselves do not move significantly, but they vibrate and pass their kinetic energy to adjacent particles.
- Vibration of Particles- When a part of a solid object is heated, the particles in that area gain energy and start vibrating more vigorously. These vibrations are then transferred to neighbouring particles.
- Efficiency Due to Solid Structure- Solids are particularly good at conducting heat because their particles are closely packed. This close packing allows energy to be transferred more efficiently from particle to particle compared to liquids or gases.
Conductivity of Materials
- Varied Conductivity- Not all materials conduct heat with the same efficiency. This variation is primarily due to the differences in their molecular structure and bonding.
- Metals as Good Conductors- Metals like copper and aluminium are excellent conductors of heat. This high conductivity is due to the presence of free electrons. In metals, some electrons are not bound to any particular atom and can move freely throughout the material. When one part of a metal is heated, these free electrons gain energy and move rapidly, transferring the heat across the metal.
- Poor Conductors – Wood and Plastic- Materials like wood and plastic are poor conductors of heat. They lack free-moving electrons and their molecular structure does not allow for efficient transfer of vibrational energy.
Conductors and Insulators
- Conductors- Conductors are materials that allow heat to pass through them easily. Copper and aluminium are common examples used in cookware, electrical wiring, and heat exchangers because of their ability to transfer heat quickly.
- Insulators- Insulators are materials that resist the flow of heat. They are used to prevent the loss or gain of heat. Common insulators include wool, fibreglass, and even air. These materials are used in building insulation, protective clothing, and thermoses to maintain temperature stability.
Activities and Experiments
- Hands-On Learning- Simple experiments, such as comparing how quickly different materials heat up, can demonstrate conduction.
- Observing Heat Transfer- Touching different materials left in the sun (like metal, stone, and wood) shows how some conduct heat more effectively than others.
Also Check – NCERT Exemplar Solutions- Class 7 Science – Chapter 3 – Heat
Convection
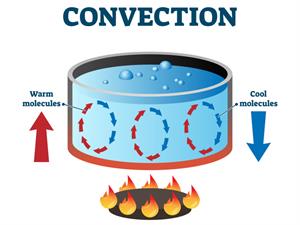
Convection is another form of heat transfer, primarily occurring in liquids and gases. This section will explore the mechanics of convection, provide practical examples, and discuss its role in natural phenomena like sea and land breezes.
Heat Transfer in Liquids and Gases
- Fluid Movement- Unlike conduction, convection involves the movement of the fluid itself. When a part of a liquid or gas is heated, it becomes less dense and rises. Cooler, denser fluid then takes its place, creating a circular motion.
- Efficiency in Fluids- Convection is an efficient way to transfer heat in fluids because it involves the actual movement of mass, carrying heat energy with it.
Explanation of Convection
- Everyday Examples- A common example of convection is the heating of water in a pot. The water at the bottom heats up, becomes less dense, and rises. The cooler water then descends to take its place, heating up in turn.
- Heating Systems- In homes, convection is used to circulate warm air. Heaters warm the air, which then rises and spreads throughout the room. As it cools, it descends and is heated again, creating a cycle.
Role in Natural Phenomena
Sea Breezes and Land Breezes
- Convection on a Large Scale- Sea and land breezes are natural phenomena that occur due to convection, which involves the movement of air caused by temperature differences.
Daytime – Sea Breeze-
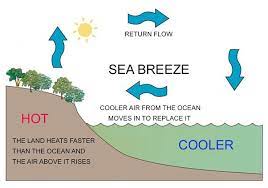
- Process- During the day, land surfaces heat up faster than water bodies. This heating causes the air above the land to warm up, become less dense, and rise.
- Air Movement- As the warm air over the land rises, a pressure difference is created. Cooler, denser air from over the sea moves in to replace the rising warm air.
- Result- This movement of air from the sea to the land creates a sea breeze, a cooling wind that can be felt near coastlines.
Nighttime – Land Breeze-
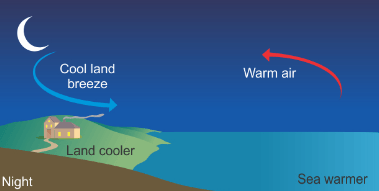
- Reverse Process- At night, the reverse occurs. Land surfaces lose heat more quickly than water. As a result, the air over the land becomes cooler and denser than the air over the sea.
- Air Movement- The cooler air over the land moves towards the sea, replacing the warmer air above the water that rises due to its lower density.
- Result- This creates a land breeze, a flow of air from the land towards the sea.
Global Weather Patterns
- Convection’s Impact- Convection is not just limited to local phenomena like sea and land breezes but also plays a crucial role in shaping global weather patterns.
- Air Movement in Atmosphere- The sun heats different parts of the Earth unevenly. This uneven heating causes variations in air temperature and density, leading to the movement of air masses.
- Formation of Wind- As warm air rises in one region, cooler air from surrounding areas moves in to replace it, creating wind patterns.
- Weather Changes- These movements of air masses, driven by convection, are fundamental in forming clouds, precipitations, and various weather conditions globally.
Also Check – Sea Breezes and Land Breezes – Class 7 Science explained in details
Also Check – Difference Between Sea Breeze And Land Breeze
Radiation
Radiation stands out as a unique method of heat transfer, characterised by its ability to occur without any intervening medium. This section delves deeper into the mechanics of radiation and its various implications in everyday life.
Heat Transfer Without a Medium
- Fundamental Nature- Unlike conduction and convection, radiation does not require a material medium to transfer heat. This means radiation can occur in a vacuum, where there are no particles to facilitate the transfer of energy.
- Mechanism of Energy Transfer- Radiation involves the emission of electromagnetic waves, which carry energy from the emitting to the receiving body.
Also Check – Conduction, Convection, and Radiation- Class 7 Science Explained
Sun as a Prime Example
- Solar Radiation- The Sun is a powerful source of radiant energy. It emits a broad spectrum of electromagnetic waves, including visible light, ultraviolet light, and infrared radiation.
- Transmission through Space- These waves traverse the vacuum of space and reach the Earth, delivering energy that warms the planet’s surface and atmosphere.
Understanding How Radiation Works
- Emission of Electromagnetic Waves- Any object with temperature above absolute zero emits radiation. The amount and type of radiation depend on the object’s temperature. For instance, a very hot object may emit visible light (like the filament in a light bulb), while cooler objects emit mostly infrared radiation.
- Spectrum of Radiation- The electromagnetic spectrum encompasses a wide range of wavelengths, from short gamma rays to long radio waves. The type of radiation an object emits falls somewhere within this spectrum.
Absorption and Emission
- Interaction with Surfaces- When these electromagnetic waves encounter a surface, they can be absorbed, reflected, or transmitted.
- Impact of Surface Properties- Dark and rough surfaces tend to absorb more radiation and thus heat up more than light or smooth surfaces. This is why wearing a black shirt on a sunny day feels hotter than wearing a white one.
- Balancing Act- Objects not only absorb radiation but can also emit it. The balance between absorbed and emitted radiation determines the object’s temperature change.
Everyday Implications
- Feeling the Sun’s Heat- We feel the heat from the Sun due to radiation. Even on a cold day, standing in direct sunlight can feel warm because of the radiated heat.
- Home Heating and Cooking- Radiation is used in household appliances like toasters and infrared heaters. These devices emit infrared radiation to cook food or heat a room.
- Technology and Industry- Radiation principles are applied in various technologies, such as solar panels, which convert solar radiation into electricity.
Energy-Efficient Building Design
This section explores how energy-efficient building design, particularly through the use of insulation and specific building materials like hollow bricks, can significantly enhance a building’s energy efficiency.
Role of Trapped Layers of Air in Insulation
- Insulation Basics- Insulation in buildings is crucial for maintaining a comfortable indoor temperature and reducing energy consumption.
- Trapped Air as an Insulator- One of the key principles of insulation is the use of trapped air layers. Air, when not allowed to circulate freely, is an excellent insulator. This is because air molecules, when stationary, minimise the transfer of heat by conduction.
- Application in Buildings- Insulation materials often contain numerous small pockets of trapped air. These pockets help prevent the flow of heat into or out of a building, keeping it warmer in winter and cooler in summer.
Using Hollow Bricks for Energy Efficiency
- Hollow Bricks Design- Hollow bricks, with their internal cavities, naturally trap air within these spaces.
- Enhanced Insulation Properties- These air pockets in hollow bricks reduce the rate of heat transfer through the walls. As a result, they help maintain a stable temperature inside the building regardless of external weather conditions.
- Additional Benefits- Besides thermal insulation, hollow bricks also provide sound insulation and reduce the overall weight of the structure, contributing to its stability and efficiency.
Clothing and Temperature
In this section, we delve into the vital role clothing plays in temperature regulation and how the colour of clothing can impact heat absorption.
Choosing Appropriate Clothing for Different Temperatures
- Temperature Regulation- Clothing acts as a personal insulation system, helping to maintain our body’s temperature balance. The type of clothing we choose depends on the environmental temperature.
- Layering in Cold Weather- In colder temperatures, wearing multiple layers helps trap body heat. Materials like wool and fleece, known for their insulation properties, are commonly used.
- Light Clothing in Warm Weather- Conversely, in warmer temperatures, light and breathable fabrics like cotton are preferred. These materials allow for air circulation and help in dissipating body heat.
Experiments on Color and Heat Absorption
- Colour Impact- The colour of clothing can significantly influence how much heat it absorbs or reflects. Dark colours tend to absorb more heat, while light colours reflect it.
- Simple Experiment- A practical experiment to understand this concept involves placing pieces of cloth of different colours under the sun. After some time, by touching them, one can feel that darker colours are warmer than lighter ones.
- Applying the Concept- This knowledge is applied in choosing clothing colours based on the weather – darker colours for colder days to absorb more heat and lighter colours for hot days to keep cool.
Keeping Warm with Wool
In this section, we’ll explore the unique insulating properties of wool and how the concept of trapped air in fabrics contributes to its effectiveness in keeping us warm.
Insulating Properties of Wool
- Natural Insulator- Wool is a natural fibre known for its excellent insulation. This is due to its unique structure and composition.
- Fibre Structure- The fibres in wool are crimped, meaning they have a natural wave or bend. This structure creates tiny pockets of air throughout the woollen fabric.
- Heat Retention- These air pockets trap body heat, preventing it from escaping into the colder external environment. This makes wool exceptionally good at keeping us warm.
Trapped Air in Fabrics
- Air as an Insulator- Air, when trapped and still, is a poor conductor of heat. It’s this property that makes trapped air an effective insulator.
- Wool’s Advantage- The crimped nature of wool fibres maximises the amount of air trapped within the fabric. More trapped air means better insulation.
- Versatility in Weather- Wool can regulate temperature due to its ability to trap air. It keeps us warm in cold conditions by retaining heat and can also be comfortable in warmer conditions due to its breathability and moisture-wicking properties.