Activity 4.5 in the chapter “Acids, Bases, and Salts” is designed to provide students with a clear understanding of the neutralisation process. This activity involves the reaction between an acid (dilute hydrochloric acid) and a base (sodium hydroxide solution), using phenolphthalein as an indicator. It’s a practical demonstration of how acids and bases react to form a neutral solution.
Also Check – What is Neutralisation? – The Basics Explained
Activity 4.5- NEUTRALISATION REACTION WITH PHENOLPHTHALEIN INDICATOR
Materials Needed
- Dilute Hydrochloric Acid (HCl)
- Sodium Hydroxide Solution (NaOH)
- Phenolphthalein Indicator
- Test tube or beaker
- Dropper
- Safety equipment- Gloves, goggles
Class 7 – Chapter 4- Acids, Bases, and Salts- An Overview of Activities
Procedure and Observations-
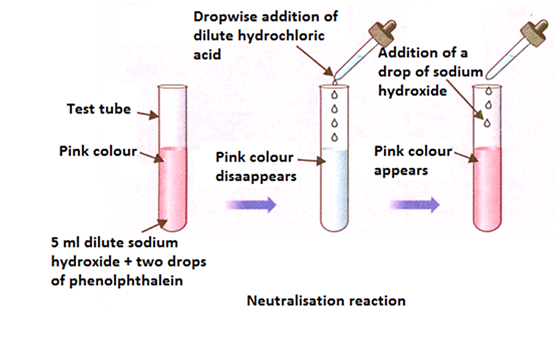
- Adding Phenolphthalein to Acid-
- Dilute hydrochloric acid is taken in a test tube.
- 2-3 drops of phenolphthalein indicator are added.
- Initially, there is no colour change observed because phenolphthalein is colourless in acidic solutions.
- Adding Sodium Hydroxide to the Acid-Indicator Mixture-
- Sodium hydroxide is added drop by drop to the acidic solution.
- A change is observed as the solution turns pink indicating that the environment inside the test tube is becoming basic.
- Adding More Hydrochloric Acid-
- When more hydrochloric acid is added to the pink solution, it turns back to colourless, indicating an acidic environment again.
Also Check – What is a Neutralisation Reaction?- Explained
Conceptual Understanding
- Neutralisation Reaction-
- Neutralisation is a chemical reaction in which an acid and a base react to form water and a salt, effectively neutralising each other’s properties.
- Role of Indicators-
- Phenolphthalein is a chemical indicator used to determine the acidity or basicity of a solution. It is colourless in acidic and neutral solutions but turns pink in basic solutions.
- Observing the End Point-
- The change in colour to pink indicates the solution has turned basic. The point at which the solution just turns pink is the end point of the neutralisation reaction.
- Acid-Base Titration-
- This activity is a simple form of titration, a method used in chemistry to determine the concentration of an acid or a base.
- Practical Implications-
- Understanding neutralisation is essential in various real-world applications, including environmental management, medicine, and manufacturing.
Learning Outcomes
- Understanding Neutralization- Students learn the concept of neutralisation and how acids and bases can cancel each other out.
- Indicator Usage- Demonstrates the use of phenolphthalein as an indicator to detect the end point of a reaction.
- Chemical Reaction Monitoring- This activity teaches students how to carefully monitor and observe chemical reactions.
- Application of Theoretical Knowledge- It bridges the gap between theoretical chemical concepts and their practical applications in a controlled environment.
Conclusion
Activity 4.5 is a crucial experiment that enhances students’ understanding of the neutralisation process, a fundamental concept in chemistry. By engaging in this practical experiment, students not only learn about the reaction between acids and bases but also gain hands-on experience in observing and recording chemical reactions. This activity reinforces their theoretical knowledge and prepares them for more advanced concepts in chemistry.
Also Check- Young Explorer’s Guide to Acids and Bases
Also Chekc – Rapid Revision – Class 7 Science – Chapter 5-Acids, Bases and Salts
Also Check – NCERT Exemplar Solutions- Class 7 Science Chapter – 5- Acids, Bases and Salts
Also Check – NCERT Solutions For Class 7 Science Chapter 5 – Acids, Bases and Salts
Also Check – Class 7- Chapter 4 – Acids, Bases and Salts – 4 Worksheets Solved and Unsolved
Class 7 science -Chapter 5-Acids, Bases and Salts – Definition and Explanation of Important Keywords
Also Check -Class 7 science -Chapter 5-Acids, Bases and Salts- Complete Notes