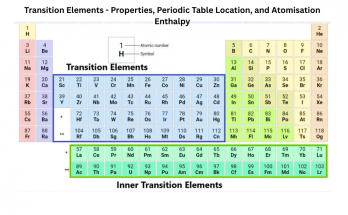
What Are Transition Elements? Properties, Periodic Table Location, and Atomisation Enthalpy
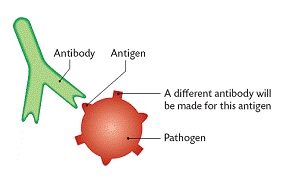
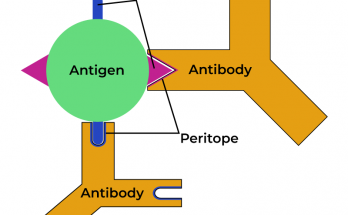
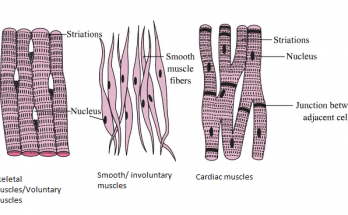
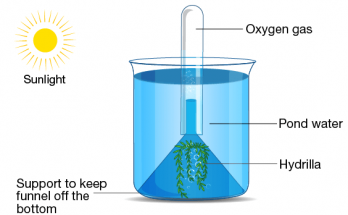
Transition elements, or d-block metals (Groups 3–12), feature partially filled d-orbitals and exhibit unique properties such as variable oxidation states, colored compounds, and catalytic activity. Located centrally on the periodic table, they have high atomisation enthalpy due to strong metallic bonding and unpaired d-electrons. Inner transition elements include lanthanides and actinides with f-orbitals.
What Are Transition Elements? Properties, Periodic Table Location, and Atomisation Enthalpy Read More