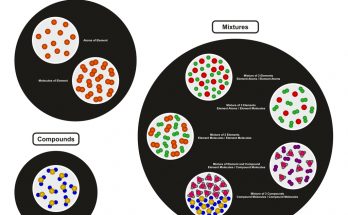
Mixtures are physical combinations of two or more substances that are not chemically combined and retain their individual properties. Based on the uniformity of composition and the visibility of components, mixtures are classified into two main types-
- Homogeneous mixtures
- Heterogeneous mixtures
Table of Contents
What is a Homogeneous Mixture?
A homogeneous mixture is one in which the components are uniformly distributed throughout the mixture. It appears as a single phase and the individual substances are not visible to the naked eye.

Characteristics of Homogeneous Mixtures-
- Uniform Composition– The mixture looks the same throughout.
- Single Phase– Only one phase is visible (solid, liquid, or gas).
- No Visible Particles– Components are mixed at the molecular or ionic level.
- Difficult to Separate– Requires advanced techniques like distillation or chromatography.
- No Tyndall Effect– Particles are too small to scatter light.
Common Examples of Homogeneous Mixtures-
- Saltwater – Salt completely dissolves in water.
- Sugar solution – Sugar dissolves uniformly in water.
- Air – A consistent mixture of gases such as nitrogen and oxygen.
- Vinegar – Acetic acid dissolved in water.
- Alloys – Metal mixtures like brass (copper and zinc), or steel (iron and carbon).
What is a Heterogeneous Mixture?
A heterogeneous mixture has a non-uniform composition and the different components remain physically distinct. The substances often exist in multiple phases and the boundaries between components are usually visible.

Characteristics of Heterogeneous Mixtures-
- Non-uniform Composition– Different parts of the mixture have different compositions.
- Multiple Phases– Two or more phases can be seen.
- Visible Components– Substances can be identified separately.
- Easily Separated– Components can be separated using filtration, decantation, sieving, or magnetism.
- May Show Tyndall Effect– If colloidal in nature, it scatters light.
Common Examples of Heterogeneous Mixtures-
- Sand in water – Sand settles at the bottom.
- Oil and water – Two layers form because they do not mix.
- Salad – Each vegetable can be seen and picked out separately.
- Milk – A colloid where fat droplets are suspended in water.
- Smoke – A mixture of solid particles in air.
Also Check – Chapter 2: Is Matter Around Us Pure?-Class 9 – Simplified notes
Comparison Table- Homogeneous vs. Heterogeneous Mixtures
| Feature | Homogeneous Mixture | Heterogeneous Mixture |
| Definition | Components are uniformly distributed and form a single phase | Components are not uniformly distributed and exist in multiple phases |
| Phases | Single-phase system | Two or more distinct phases |
| Particle Distribution | Evenly mixed at molecular level | Unevenly mixed, components often visible |
| Visibility | Components are not visible separately | Components are visible to the naked eye or under a microscope |
| Separation | Not easily separated by physical means | Easily separated by physical methods like filtration or decantation |
| Tyndall Effect | Not observed | Observed in colloidal heterogeneous mixtures |
| Examples | Saltwater, vinegar, air, alloys | Oil in water, sand in water, salad, milk, smoke |
Detailed Examples
Example of Homogeneous Mixture- Sugar in Water
When sugar dissolves in water, it spreads evenly throughout the solution. The mixture appears as a single liquid phase and sugar is no longer visible. This mixture does not scatter light, indicating no Tyndall effect.
Example of Heterogeneous Mixture- Oil and Water
When oil is poured into water, it floats on top, forming two distinct layers. The boundary between the oil and water is clearly visible and the two substances can be separated by decantation, making this a heterogeneous mixture.
Additional Key Concepts
- Colloids– A type of heterogeneous mixture where particles do not settle down and are dispersed in another medium (e.g., milk, fog, jelly).
- Suspensions– Mixtures with larger particles that settle over time (e.g., muddy water).
- Homogenization– A process used to make certain mixtures like milk uniform by breaking down fat molecules.
- Real-life applications– Air (a homogeneous mixture) is essential for respiration, while concrete (a heterogeneous mixture) is widely used in construction.
Frequently Asked Questions
1. What is the main difference between homogeneous and heterogeneous mixtures?
A homogeneous mixture has a uniform composition and appears as one phase, while a heterogeneous mixture has visible differences and multiple phases.
2. Is milk a homogeneous or heterogeneous mixture?
Milk is a colloidal heterogeneous mixture. Though it may appear uniform, under a microscope, fat globules are visible.
3. Can we separate the components of a homogeneous mixture?
Yes, but it usually requires specialized techniques such as distillation, evaporation, or chromatography, as the components are not visibly distinct.