DECANTATION Definition
Decantation is the process of pouring out a clear liquid from a vessel (after sedimentation) without disturbing the sediment (heavy insoluble settled particles).
Process of DECANTATION
There are some mixtures which contain insoluble solid particles suspended in a liquid. The solid particles which are insoluble in a liquid can be separated by decantation.
For example, a mixture of sand and water contains sand particles suspended in water. Sand can be separated from the sand-water mixture by the method of decantation.
Process of decantation is as follows-
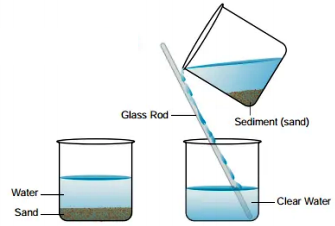
- The sand and water mixture is taken in a beaker. It contains sand particles scattered throughout water.
- Allow the mixture of sand and water to stand undisturbed for some time. On keeping, the heavier sand particles will settle down at the bottom of the beaker. This layer of sand is called Sediment.
- The deposition of sand at the bottom of the beaker is called sedimentation. When the sand settles down, clear water is left above the layer of sand.
- We pour the clear water into another beaker gently with the help of a glass rod, without disturbing the sediment of sand .This process is Decantation.
- When all the clear water has been poured out, only sand is left at the bottom of the beaker. In this way, a mixture of sand and water has been separated into ‘sand’ and ‘clear water’ by Decantation.
Also Check – FILTRATION- Definition, Process, Examples and Limitations
Examples of DECANTATION
Rice and pulses are always washed before cooking. When we add water to rice or pulses taken in a vessel, then the impurities such as dust and soil particles get separated and go into water. Due to this the water becomes a little dirty. The rice or pulses, being heavy, remain at the bottom of the vessel and dirty water is separated by decantation by tilting the vessel to one side. Thus, when rice and pulses are washed with water before cooking, then the water is separated from them by decantation.

Decantation is also used to separate water from some vegetables after boiling. The method of decantation can also be used for separating two immiscible liquids. For example, water and oil are two immiscible liquids which can be separated by decantation.
A mixture of water and oil is allowed to stand in a beaker. Since water and oil are immiscible, they form two separate layers in the beaker. Water, being heavier, forms the lower layer and oil, being lighter, forms the upper layer in the beaker. We can decant off the upper layer of oil into another beaker carefully. Water will be left behind.
The separation of two immiscible liquids by decantation is not very satisfactory.
Limitations of Decantation
The process of decantation can be used for separation only when the solid does not dissolve in the liquid. Decantation cannot be used for separation if the solid dissolves in the liquid.
For example, sugar dissolves in water, so a mixture of sugar and water cannot be separated by the method of decantation.
Decantation cannot be used for separating two miscible liquids (which mix with each other).
For example, alcohol and water are miscible with each other (which mix to form a single layer). So, a mixture of alcohol and water cannot be separated by decantation. Similarly, a mixture of kerosene and petrol cannot be separated by decantation. This is because they are miscible liquids.
