The Mixing Lead Nitrate and Potassium Iodide Solutions activity in the chemistry curriculum of Class 10 is an excellent demonstration of a double displacement reaction, a type of chemical reaction where parts of two ionic compounds are exchanged and form two new compounds. This experiment involves mixing aqueous solutions of lead nitrate and potassium iodide, leading to the formation of lead iodide, a yellow precipitate, and potassium nitrate, which remains in solution.
Activity 1.2 Mixing Lead Nitrate and Potassium Iodide Solutions
Materials Needed-
- Lead nitrate solution- An aqueous solution of lead nitrate, acting as one of the reactants.
- Potassium iodide solution- An aqueous solution of potassium iodide, serving as the second reactant.
- Safety equipment- Protective goggles, lab coat, and gloves to ensure safety from potential hazards of handling lead compounds.
- Two beakers or test tubes- For mixing the solutions.
Steps of the Activity-
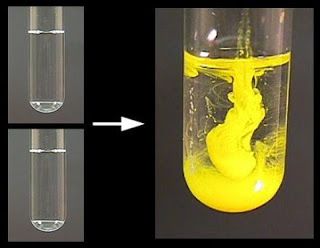
- Preparation- Measure equal volumes of lead nitrate and potassium iodide solutions in separate beakers or test tubes.
- Combination- Pour the potassium iodide solution into the lead nitrate solution, mixing them together.
- Observation- Look for the formation of a yellow precipitate, which is lead iodide.
What to Look For-
- The immediate formation of a bright yellow precipitate, indicating the formation of lead iodide.
- The solution may also become clearer as the precipitate settles, showing the separation of the solid from the liquid phase.
Why It Happens-
- This reaction is a classic example of a double displacement reaction-
Pb(NO3)2(aq) + 2KI(aq)→PbI2(s) + 2KNO3(aq)Pb(NO3)2(aq) + 2KI(aq)→PbI2(s) + 2KNO3(aq).
- The lead (Pb^2+) ions from lead nitrate react with the iodide (I^-) ions from potassium iodide to form solid lead iodide, while the potassium (K^+) and nitrate (NO3^-) ions remain in solution as potassium nitrate.
Importance of the Activity-
- Demonstrates the concept of ionic reactions in solution and the formation of precipitates.
- Highlights the principles of solubility and the role of ionic compounds in chemical reactions.
- Provides a visual representation of chemical changes and the conservation of mass through the formation of new substances.
This activity is valuable for understanding the dynamics of chemical reactions, the interaction of ionic compounds, and the visual and tangible aspects of precipitate formation, making it a significant educational experience in the study of chemistry.