The “Burning Magnesium Ribbon” activity in the chemistry curriculum of Class 10 demonstrates a fundamental chemical process known as a combination reaction. This experiment involves the reaction of magnesium metal with oxygen from the air to form magnesium oxide, a new compound, showcasing how elements combine to form more complex substances.
Activity 1.1 Burning of a Magnesium Ribbon in air
Materials Needed:
- Magnesium ribbon: A thin strip of magnesium metal that serves as the reactant.
- Safety equipment: Protective goggles, lab coat, and gloves to ensure safety from the intense light and heat produced.
- Ignition source: A Bunsen burner or a matchstick to ignite the magnesium ribbon.
- A container or a clamp to hold the magnesium ribbon.
Steps of the Activity:
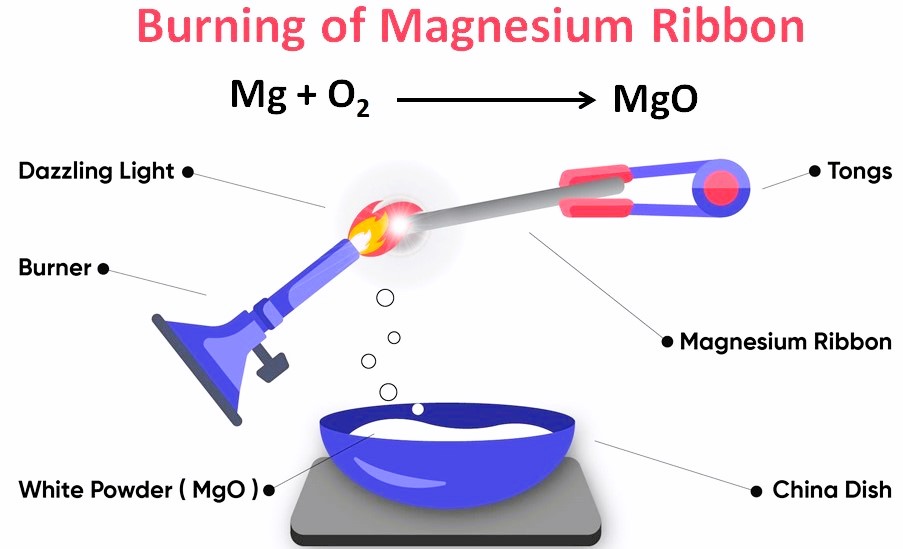
- Preparation: Clean the magnesium ribbon with sandpaper to remove any oxide layer, ensuring a more vigorous reaction.
- Ignition: Hold the magnesium ribbon with tongs or a clamp and ignite it using the Bunsen burner or matchstick, keeping it at a safe distance to avoid the bright light harming the eyes.
- Observation: Watch as the magnesium burns with a brilliant white light, forming white ash, which is magnesium oxide.
Also Check – Why should a magnesium ribbon be cleaned before burning in air?
What to Look For:
- The intense white light and heat indicate the exothermic nature of the reaction.
- The white ash formed, which is magnesium oxide, showing the transformation from a metal to an oxide.
Why It Happens:
- The reaction is an example of oxidation, where magnesium loses electrons to oxygen, forming MgO. This is a combination reaction where two elements combine to form a single product.
Importance of the Activity:
- Demonstrates the principle of combination reactions in chemistry.
- Highlights the concept of oxidation and reduction.
- Engages with the conservation of mass principle, as the mass of magnesium and oxygen before the reaction equals the mass of magnesium oxide produced.
This activity not only illustrates the chemical reaction process but also emphasises safety in chemical experimentation, the conservation of mass, and the transformation of substances at a molecular level, making it a comprehensive educational experience.