The Formation of Slaked Lime activity in the chemistry curriculum of Class 10 showcases a combination reaction where calcium oxide (quicklime) reacts with water to form calcium hydroxide (slaked lime). This is a critical process in the construction industry for making mortar and plaster.
Activity 1.4 Formation of slaked lime by the reaction of calcium oxide with water
Materials Needed-
- Calcium oxide- A white or greyish-white solid compound.
- Water- To react with calcium oxide.
- Safety equipment- Goggles, gloves, and lab coat to protect from the exothermic reaction heat.
- A container- Preferably a beaker or a mixing bowl.
Steps of the Activity-
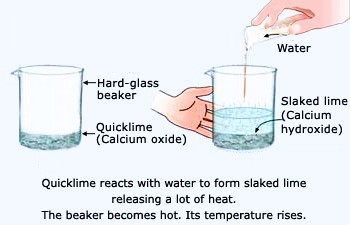
- Preparation- Place a small amount of calcium oxide in the container.
- Combination- Slowly add water to the calcium oxide.
- Observation- Notice the exothermic reaction producing heat and forming a fine white powder, calcium hydroxide.
What to Look For-
- The release of heat, indicating an exothermic reaction.
- The formation of a white, powdery substance, which is slaked lime.
Why It Happens-
- Calcium oxide combines with water in a combination reaction, forming calcium hydroxide and releasing heat.
The ‘Formation of Slaked Lime’ activity demonstrates a combination reaction, detailed by the equation-
CaO (s) + H₂O (l) → Ca(OH)₂ (aq)
In this exothermic reaction, calcium oxide (quicklime) combines with water to form calcium hydroxide (slaked lime), releasing heat. The experiment highlights the concepts of combination reactions, the exothermic nature of certain chemical processes, and their practical applications, such as in construction for making mortar and plaster, requiring careful handling due to the heat generated.
Importance of the Activity-
- Demonstrates a key chemical process in the production of building materials.
- Highlights the exothermic nature of some combination reactions.
This activity provides insight into the practical applications of chemical reactions in everyday materials and the safety considerations involved in handling exothermic reactions.