Table of Contents
Respiration vs Combustion (Burning)
In the vast and intricate realm of science, there exist countless processes that fuel the world around us. Two such pivotal processes are respiration and combustion. At first glance, they might seem unrelated—one tied to living organisms and the other to the flames of a fire. However, both play a foundational role in producing energy, albeit in very different contexts.
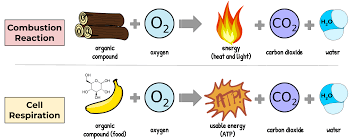
Respiration is a life-sustaining biochemical process. Every living cell, from the cells in our muscles to the tiniest bacteria, relies on respiration to convert nutrients into usable energy. Think of it as nature’s intricate energy-generation system, tailored to support life. Every time you run, think, or even simply breathe, it’s the energy from respiration at play. The process breaks down organic molecules, like glucose, in the presence of oxygen to release energy, water, and carbon dioxide. This energy is then stored in a molecule called ATP (Adenosine Triphosphate), which powers countless cellular activities.
On the other hand, combustion—often recognized by the familiar sight of flames—is a physico-chemical process. It’s not limited to living organisms. When we light a campfire, ignite fuel in an engine, or watch fireworks explode in the sky, we witness combustion. This process involves the rapid reaction of a substance with an oxidising agent, usually oxygen, producing heat, light, and often, gases like carbon dioxide. It’s the science behind many modern technologies, from vehicles to power plants.
11 Difference between Respiration and Combustion (burning) – Tabular form
| Aspect | Respiration | Combustion |
|---|---|---|
| Nature of Process | Cellular process | Non-cellular process |
| Type of Process | Biochemical process | Physico-chemical process |
| Rate of Process | Slow process | Rapid process |
| Control | Series of stepwise oxidation under biological control | Uncontrolled single-step oxidation |
| Enzymatic Requirement | Each step requires a specific enzyme | No enzymes are involved |
| Temperature | Occurs at body temperature | Takes place at higher temperatures (typically at the ignition point of the substrate) |
| Temperature Effect | Body temperature remains constant | Temperature rises spontaneously |
| Energy Liberation | Energy is released primarily as ATP and some as heat | Energy is released mainly in the form of heat and light |
| Light Production | No light is produced | Light is emitted during the process |
| Intermediates Production | Produces many intermediates useful for synthesising organic compounds | No intermediates are produced |
| Reaction | C6H12O6+6O2→6CO2+6H2O+energy (ATP) | Combustion (of glucose as an example) – C6H12O6+6O2→6CO2+6H2O+heat and light |
Differences between Respiration and Combustion Explained in Detail
1. Nature of Process-
Respiration-
- Cellular Process- Respiration happens at a microscopic level inside the cells of living organisms. Within these cells, mitochondria—often referred to as the powerhouse of the cell—carry out the steps of respiration.
- Purpose and Impact- Through respiration, cells harness energy from organic molecules (like glucose) to support bodily functions, from moving muscles to thinking thoughts.
Combustion-
- Non-cellular Process- Combustion is an external process that takes place outside the confines of cell walls. It isn’t dependent on the intricacies of cellular structures or mechanisms.
- Manifestations- Everyday examples include burning of wood, ignition of fuels in engines, or even the simple act of lighting a candle.
2. Type of Process-
Respiration-
- Biochemical Nature- The term “biochemical” signifies that this process is both biological (it happens in living organisms) and chemical (involving chemical reactions). During respiration, glucose is broken down chemically in the presence of oxygen, releasing energy.
- Outcomes- The end products of respiration are carbon dioxide and water, both of which are expelled by organisms as waste products.
Combustion-
- Physico-chemical Nature- While combustion too involves chemical reactions, the “physico” prefix indicates the importance of physical conditions like temperature and pressure in facilitating the process.
- Reactions- Combustion typically involves a fuel reacting quickly with an oxidant, leading to the release of energy in the form of heat and often light.
3. Rate of Process-
Respiration-
- Gradual Release- The energy derived from respiration is released at a steady pace, allowing cells to have a consistent energy supply. This steady release is crucial for maintaining cellular functions without overwhelming the cell with too much energy at once.
Combustion-
- Swift Reaction- In stark contrast, combustion is swift and intense. A lit matchstick or ignited gasoline can release a large amount of energy in seconds. This rapidity is useful for immediate energy needs, like heating a room or propelling a vehicle.
4. Control-
Respiration-
- Regulated Oxidation- The body meticulously manages respiration through various enzymes and pathways. This ensures that energy release is consistent and safe, avoiding any cellular damage.
Combustion-
- Unregulated Oxidation- Combustion lacks the biological control mechanisms inherent in respiration. Instead, it’s primarily governed by external factors like the presence of oxygen and ignition sources.
5. Enzymatic Requirement-
Respiration-
- Enzyme-driven- Every step of respiration is catalyzed by specific enzymes. These enzymes are proteins that speed up reactions, ensuring that respiration occurs efficiently at body temperature.
Combustion-
- Absence of Enzymes- Combustion reactions don’t rely on enzymes. Instead, they depend on certain conditions, such as the right mix of fuel and oxidant, as well as a specific ignition temperature.
6. Temperature-
Respiration-
- Consistency at Body Temperature- Respiration is an adaptation that allows organisms to produce energy efficiently at a consistent temperature. For humans, this process is finely tuned to occur around 37°C, which is the average body temperature.
- Cellular Benefits- By occurring at body temperature, respiration ensures that the cellular environment remains stable, which is crucial for the function and longevity of cells.
Combustion-
- Varied Ignition Points- The temperature at which combustion begins, known as the ignition point, varies depending on the material. For instance, paper has a much lower ignition temperature than metal. Typically, these ignition points are significantly higher than any biological body temperature.
7. Temperature Effect-
Respiration-
- Thermal Homeostasis- Even though respiration releases energy, organisms have evolved mechanisms to dissipate excess heat. In humans, sweating, vasodilation (widening of blood vessels), and other mechanisms prevent overheating, maintaining thermal homeostasis.
Combustion-
- Temperature Surge- When materials combust, there’s a distinct and rapid increase in temperature. The heat generated can be felt when we warm our hands near a fire or when we touch an overheated engine.
8. Energy Liberation-
Respiration-
- ATP Production- Respiration’s primary purpose is to produce adenosine triphosphate (ATP), a molecule that acts as the primary energy currency in cells. While a minor amount of heat is released, the primary focus is on synthesising ATP.
Combustion-
- Heat and Light- The primary products of combustion are heat and, in many cases, light. This is evident when we bask in the warmth of a campfire and enjoy its radiant glow.
9. Light Production-
Respiration-
- A Silent Process- Respiration, being a biochemical process, doesn’t produce visible light. While energy is continuously being released and used, there’s no accompanying luminosity.
Combustion-
- Flames and Brightness- One of the most distinguishable features of combustion is the production of light, such as the flames of a burning log or the bright burst from fireworks.
10. Intermediates Production-
Respiration-
- Biochemical Intermediates- As cells break down glucose, several intermediary molecules are produced. These can be further used by the cell to synthesize amino acids, lipids, and other vital organic compounds. This multi-step, multi-product approach is a hallmark of the complexity of cellular metabolism.
Combustion-
- Limited Byproducts- Combustion’s primary aim is to release energy rapidly. The process doesn’t produce a variety of intermediates like respiration does. Instead, combustion typically results in gases like CO2 and water vapour. Solid residues, like ash, might remain, depending on the burned material.
11.Reaction
Respiration-
The cellular respiration equation represents the metabolic pathway through which cells harvest energy from organic compounds like glucose. The process is complex and is carried out in several steps, primarily in mitochondria for eukaryotic cells-
- Glycolysis- Occurs in the cell’s cytoplasm and breaks down glucose into two molecules of pyruvate. ATP is produced, but it’s a modest amount.
- Krebs Cycle (Citric Acid Cycle)- Takes place in the mitochondria and further breaks down the products of glycolysis to produce energy-rich molecules like NADH and FADH2.
- Electron Transport Chain- Located in the inner mitochondrial membrane. Here, electrons are transferred between protein complexes, and oxygen acts as the final electron acceptor, producing water. The energy from this process is used to produce ATP.
The primary goal of respiration is to produce ATP, the cellular currency of energy.
Combustion
Combustion, often referred to as burning, is a high-temperature exothermic reaction between a fuel and an oxidant, usually oxygen in the air. For our example, we’ve used glucose as the fuel, but it could be many other substances, including hydrocarbons (like gasoline), wood, paper, etc.
When glucose combusts, it reacts with oxygen to produce carbon dioxide and water. Unlike respiration, which occurs in controlled steps and aims to produce ATP, combustion happens rapidly, releasing energy in the form of heat and light. In real-world applications, the combustion of various fuels powers engines, provides heat for homes, and is the principle behind fireworks.
Despite their apparent similarities in the basic equations, the underlying processes and outcomes of respiration and combustion are distinct, with each playing a crucial role in biology and technology, respectively.
Also Check – 13 Important Differences between Photosynthesis and Respiration
Also Check – 9 Important Diffrences Between Aerobic and Anaerobic Respiration
Also Check – Respiration Class 10 Notes
Also Check – Respiration in Human Beings – Class 10
Also Check – Importance Of Respiration
Also Check – Respiration in Animals
One Comment on “11 Differences between Respiration and Combustion (Burning)”