Question-Why are decomposition reactions called the opposite of combination reactions? Write an equation for this reaction.
Answer-
Decomposition and combination reactions are considered opposites due to the fundamental difference in their reaction processes.
Decomposition Reactions-
In a decomposition reaction, a single compound breaks down into two or more simpler substances, often requiring an external energy source like heat or electricity.
Examples of Decomposition Reaction-
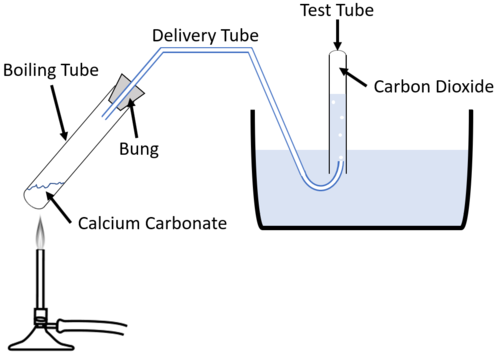
Calcium Carbonate Decomposition-
Reaction- When calcium carbonate (CaCO₃) is heated, it decomposes into calcium oxide (CaO) and carbon dioxide (CO₂).
Equation-
CaCO₃(s) → heat CaO(s) + CO₂(g)
- Description- Here, calcium carbonate undergoes thermal decomposition, resulting in calcium oxide and carbon dioxide.
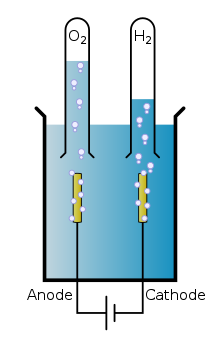
Water Molecule Decomposition-
Reaction- Passing electric current through water (H₂O) decomposes it into oxygen (O₂) and hydrogen (H₂).
Equation-
2H₂O(l) → Electric current 2H₂(g) + O₂(g)
- Description- In this electrolysis process, water is decomposed into hydrogen and oxygen gases.
Combination Reactions-
Conversely, combination reactions involve the merging of two or more substances to form a new, single substance, often releasing energy in the process.
Examples of Combination Reaction-
Formation of Slaked Lime-
Reaction- Calcium oxide (CaO) reacts with water (H₂O) to form calcium hydroxide [Ca(OH)₂], also known as slaked lime, along with the release of heat.

Equation-
CaO(s) + H₂O(l) → Ca(OH)₂(aq) + heat
- Description- This exothermic reaction involves the combination of calcium oxide and water to produce slaked lime.
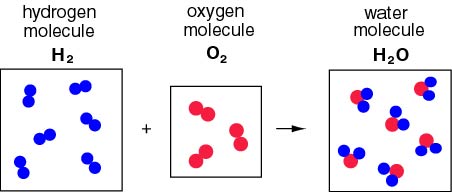
Formation of Water-
Reaction- Hydrogen gas (H₂) combines with oxygen gas (O₂) to form water (H₂O).
Equation-
2H₂(g) + O₂(g) → 2H₂O(l)
- Description- In this reaction, hydrogen and oxygen gases combine to produce water, showcasing the essence of a combination reaction.
Through these examples, it shows that decomposition reactions, characterised by the breakdown of a compound into simpler substances, are the opposite of combination reactions, where simpler substances combine to form a more complex product.