The property of matter used to measure temperature is thermal expansion.
Thermal expansion is the tendency of matter to expand in volume when its temperature increases. This happens because heating increases the kinetic energy of the particles in a substance. As a result the particles move more vigorously and the average space between them increases, causing the substance to expand. While the volume changes, the mass of the substance remains nearly constant.
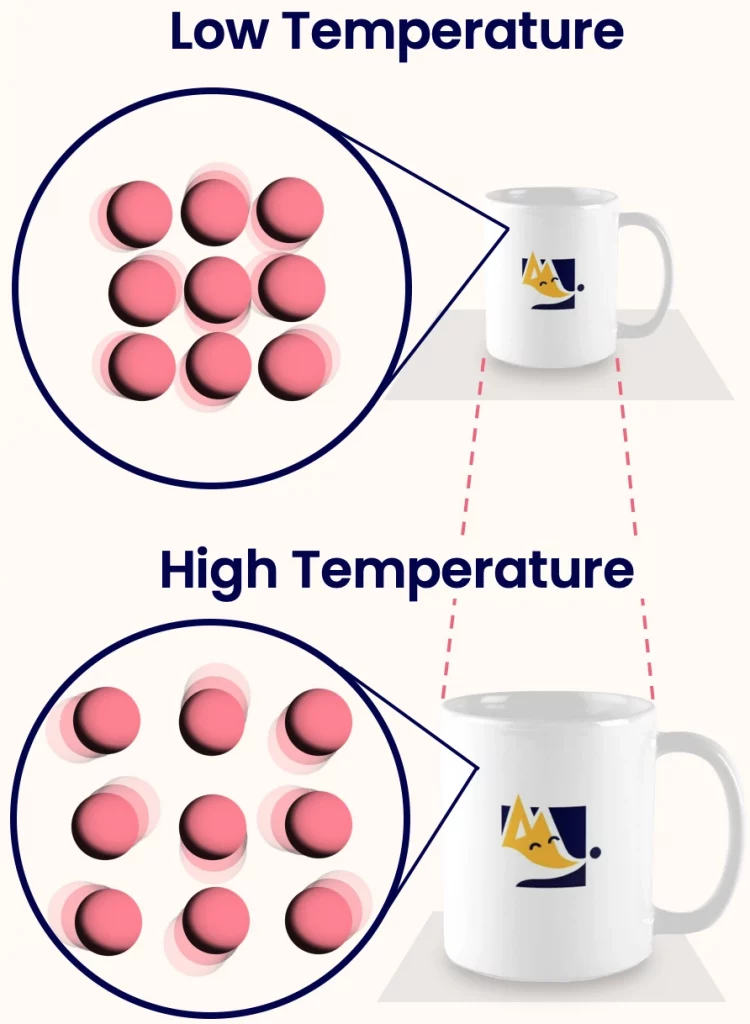
Why Thermal Expansion is Used to Measure Temperature:
Temperature is defined as the degree of hotness or coldness of a body and is directly related to the average kinetic energy of the particles in a substance. When a substance is heated:
- Its particles move faster.
- This causes an increase in the space between particles.
- The overall size or volume of the substance increases.
This predictable change in size with temperature is what makes thermal expansion a measurable property, and hence, it is used in temperature measuring instruments.
Also Check – Properties of Matter: Physical, Chemical, and Functional Explained With Examples
Examples of Thermal Expansion in Measuring Temperature:
- Liquid-in-glass Thermometers:
- These thermometers contain mercury or alcohol.
- When temperature rises, the liquid expands and rises in a narrow tube.
- When temperature falls, the liquid contracts and moves down.
- The expansion of the liquid is calibrated against a temperature scale.
- Bimetallic Strip in Thermostats:
- A bimetallic strip is made of two metals with different expansion rates.
- When heated, the metals expand differently and cause the strip to bend.
- This bending is used to detect or control temperature in devices like electric irons or heaters.
- Gas Thermometers:
- In these, the expansion or pressure change of a fixed amount of gas is used to determine temperature.
- Gases expand more significantly with heat than solids or liquids.
Relation to States of Matter:
- Solids expand slightly with temperature. The expansion is linear and small but measurable.
- Liquids expand more than solids, which is why liquids like mercury and alcohol are used in thermometers.
- Gases expand the most and follow laws such as Charles’s Law and Gay-Lussac’s Law, which relate volume and pressure to temperature.
Effect of Temperature on Matter:
- At low temperatures, matter contracts. For example, water freezes to ice at 0°C.
- At high temperatures, matter expands. Water boils to form steam at 100°C.
- These changes are called phase transitions and are extreme examples of how temperature affects matter.
- Even without a change of state, matter continuously expands with increasing temperature, which is the principle behind using thermal expansion to measure it.
The measurement of temperature relies on the thermal expansion of matter, which is the result of increased kinetic energy and increased particle spacing. Since this expansion is predictable and measurable, it forms the basis of almost all conventional thermometric methods. Therefore, thermal expansion is the key property of matter used to measure temperature.