Table of Contents
What is Sublimation?
Sublimation is the process in which a solid changes directly into a gas without passing through the liquid phase. This means that a substance transitions from a solid state to a gaseous state without melting into a liquid in between.
This phenomenon occurs under specific conditions of temperature and pressure and is typically observed in substances that have weak intermolecular forces, allowing them to bypass the liquid phase when they gain enough energy.
How Does Sublimation Work?
At the molecular level, substances are made up of tiny particles (atoms or molecules) that are held together by intermolecular forces. In most cases, heating a solid provides energy that first weakens these forces to turn the solid into a liquid and then further weakens them to transform the liquid into a gas.
In sublimation, certain substances receive enough energy to break their intermolecular bonds completely, allowing them to transition directly into the gaseous phase.
This usually happens in conditions of-
- Low atmospheric pressure, which allows molecules to escape easily.
- Specific temperatures, at which the solid’s particles gain sufficient kinetic energy to jump directly to the gas phase.
Examples of Sublimation

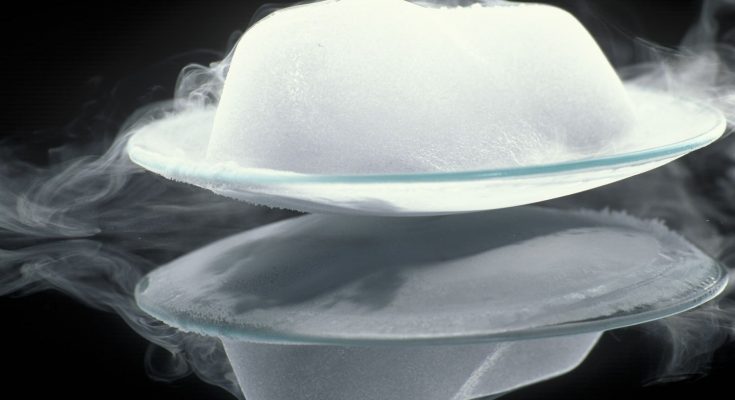
- Dry Ice (Solid CO₂) → Carbon Dioxide Gas
- Dry ice is a frozen form of carbon dioxide. When exposed to normal room temperature, it does not melt into liquid CO₂. Instead, it directly turns into carbon dioxide gas.
- That’s why dry ice is often used to create fog effects in movies and stage performances.
- Camphor or Naphthalene Balls → Vapors
- Camphor and mothballs (made of naphthalene) sublimate over time. That’s why if you leave naphthalene balls in your cupboard, they slowly disappear without forming a liquid.
- This property makes them useful for keeping insects away from clothes.
- Iodine Crystals → Purple Gas
- When iodine crystals are heated, they directly turn into a violet-colored gas without melting into a liquid.
- This is often demonstrated in chemistry labs to illustrate sublimation.
- Snow or Ice in Cold, Dry Conditions
- In extremely cold and dry conditions (such as in Antarctica or high-altitude areas), snow and ice can sublimate directly into water vapor without melting.
Applications of Sublimation
Sublimation is crucial in many scientific and industrial applications. Here are some important real-world uses-
- Sublimation Printing
- This process is widely used in printing designs on T-shirts, mugs and other materials.
- In sublimation printing, solid dyes are heated to directly form a gas, which then bonds with the material’s fibers, creating long-lasting prints.
- Freeze Drying (Lyophilization) in Food & Medicine
- Sublimation is used to remove water from food and medicine without going through the liquid phase.
- Example- Freeze-dried coffee is made by freezing coffee and then sublimating the ice under vacuum conditions.
- Air Fresheners
- Some air fresheners work using sublimation, where solid fragrance substances directly convert into vapor to spread the scent in a room.
- Purification of Substances
- In chemistry, sublimation is used to purify substances by heating them and collecting the vapor to separate impurities.
- This method is useful for purifying substances like iodine and camphor.
- Space Science (Sublimation of Comets)
- Comets experience sublimation when they approach the Sun. The heat causes frozen gases in the comet to directly turn into vapor, creating a glowing “tail” seen from Earth.
What Will Students Learn Beyond This in Higher Standards?
When students move to higher levels of education, they will learn about-
- Phase Diagrams– How temperature and pressure determine whether a substance will sublimate, melt, or boil.
- Triple Point– A unique condition where solid, liquid and gas phases coexist.
- Thermodynamics of Sublimation– The energy changes involved in phase transitions.
- Industrial Applications– Advanced uses in pharmaceuticals, food preservation and material science.
- Surface Chemistry and Vacuum Sublimation– Used in making semiconductors and coatings.
Demonstration Experiments to Show Sublimation
These simple experiments can be performed safely in a classroom or at home (under supervision) to help students visualize sublimation.
Experiment 1- Dry Ice Sublimation (Safe Fog Effect)

Materials Needed-
- Dry ice (solid CO₂) – available at some grocery stores
- A bowl or a beaker
- Warm water
- Gloves and safety goggles (important for handling dry ice)
Procedure-
- Place a small piece of dry ice in a bowl.
- Pour warm water over the dry ice.
- Observe how the dry ice sublimates into carbon dioxide gas, creating a dense fog.
What’s Happening?
- Dry ice does not melt into liquid CO₂ but directly turns into gas.
- The fog is created because the gas cools down the surrounding air, causing water vapor to condense.
Applications Related to This Experiment-
- Special effects in movies and stage performances.
- Preserving food and biological samples.
Experiment 2- Sublimation of Camphor or Mothballs
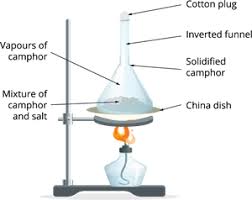
Materials Needed-
- Camphor (or mothballs made of naphthalene)
- A metal spoon or aluminum foil
- A candle or spirit lamp
- Tongs (to hold the spoon)
- A glass beaker (optional, for covering the setup)
Procedure-
- Place a small piece of camphor or a mothball in a metal spoon.
- Hold the spoon over the candle flame using tongs.
- Observe as the solid disappears and a white vapor is released.
- If you cover the setup with a glass beaker, you may see the vapor condense back into small solid particles on the glass.
What’s Happening?
- The camphor or naphthalene sublimes directly into gas without turning into a liquid.
- If the vapor cools, it turns back into tiny solid crystals.
Applications Related to This Experiment-
- Air fresheners work on this principle.
- Mothballs are used in storage areas to prevent insect damage.
Also Check in Details Sublimation Experiment with Camphor
Experiment 3- Sublimation of Iodine (Color Change Experiment)

Materials Needed-
- Iodine crystals (available in chemistry labs)
- A glass beaker
- A glass cover or watch glass
- Heat source (Bunsen burner or candle)
- Ice cubes (for optional condensation observation)
Procedure-
- Place a few iodine crystals in a beaker.
- Cover the beaker with a watch glass or another glass surface.
- Gently heat the iodine using a Bunsen burner.
- Observe the formation of purple vapors.
- If you place ice cubes on top of the watch glass, the vapor will condense back into solid iodine.
What’s Happening?
- Iodine crystals sublime when heated, turning into purple gas.
- Cooling the gas allows it to deposit back into solid iodine, a process called deposition (opposite of sublimation).
Applications Related to This Experiment-
- Used in chemistry for purifying iodine.
- Demonstrates how substances can transition between phases.
Why Are These Experiments Important?
- They visually demonstrate phase transitions without needing complex lab equipment.
- Students connect science with real-world applications like dry ice, air fresheners and mothballs.
- They prepare students for advanced chemistry topics such as phase diagrams, thermodynamics and industrial applications.
Higher-Level Concepts Related to Sublimation for Pre-University Students
As students progress to higher standards, they will explore more advanced concepts related to sublimation. These concepts integrate chemistry, physics and real-world applications, providing a deeper understanding of phase transitions.
1. Phase Diagrams & Triple Point
Concept-
- A phase diagram is a graphical representation of the different states (solid, liquid, gas) of a substance under various temperature and pressure conditions.
- The triple point is a specific temperature and pressure where a substance can exist simultaneously in all three states (solid, liquid, gas).
- Some substances, like carbon dioxide, sublimate at normal atmospheric pressure because their triple point is above atmospheric pressure.
Example-
- Dry Ice (CO₂) sublimation happens because at standard atmospheric pressure, CO₂ cannot exist as a liquid—it only transitions between solid and gas.
Applications-
- Used in climate science to understand water phase transitions in Earth’s atmosphere.
- Critical in cryogenic storage and industrial gas handling.
2. Enthalpy of Sublimation & Thermodynamics
Concept-
- The enthalpy of sublimation (ΔH_sub) is the amount of energy required to convert 1 mole of a solid into a gas.
- The equation- ΔHsub=ΔHfusion+ΔHvaporization\Delta H_{\text{sub}} = \Delta H_{\text{fusion}} + \Delta H_{\text{vaporization}}ΔHsub=ΔHfusion+ΔHvaporization shows that sublimation energy is the sum of fusion (melting) and vaporization energies.
Example-
- Iodine (I₂) sublimation requires energy to break molecular interactions directly from solid to gas.
Applications-
- Used in energy calculations for chemical engineering and space science.
- Helps in designing cooling systems for aerospace technology.
3. Sublimation in Space Science
Concept-
- Sublimation plays a key role in the behavior of celestial bodies, particularly comets and asteroids.
- As comets approach the Sun, the ice in them sublimates, creating the glowing tail seen from Earth.
Example-
- The Halley’s Comet tail is a result of sublimation, where frozen gases turn directly into vapor under solar radiation.
Applications-
- Used to study the origins of celestial bodies and space weathering processes.
- Important in the design of satellites and space probes that operate in extreme temperatures.
4. Industrial Sublimation (Purification of Substances)
Concept-
- Vacuum sublimation is a method used to purify substances by converting solids into gases and condensing them back into purified solids.
- It is an efficient way to remove impurities.
Example-
- Purification of organic compounds like caffeine and pharmaceuticals.
Applications-
- Used in the pharmaceutical industry to obtain highly pure drugs.
- In semiconductor manufacturing, sublimation helps in producing high-purity silicon wafers.
5. Freeze-Drying (Lyophilization) in Food & Medicine
Concept-
- Freeze-drying involves sublimation of ice under low pressure to remove moisture from products without damaging their structure.
- This process preserves food and biological materials for a long time.
Example-
- Instant coffee production and vaccine preservation.
Applications-
- In medicine, used to preserve blood plasma, vaccines and delicate proteins.
- Used by astronauts to carry lightweight freeze-dried food in space.
6. Sublimation in Textile & Printing Industry
Concept-
- Sublimation printing uses heat to turn solid dyes into gas, which then embeds into fabric fibers, creating permanent prints.
Example-
- T-shirt sublimation printing produces long-lasting designs that don’t fade.
Applications-
- Used in making high-quality sportswear, mugs and banners.
- Helps in creating custom branding materials.
7. Atmospheric Sublimation & Climate Science
Concept-
- In extremely cold and dry environments, snow and ice can sublimate directly into vapor without melting.
- This process is crucial in glacial melting and water cycle dynamics.
Example-
- Glaciers in Antarctica lose mass partly due to sublimation.
Applications-
- Helps in predicting climate change and global warming effects.
- Important in meteorology for studying cloud formation.
8. The Role of Sublimation in Nanotechnology
Concept-
- Physical vapor deposition (PVD) uses sublimation to deposit thin films on surfaces, a key process in nanotechnology.
Example-
- Manufacturing of microchips and solar cells using sublimation-based coatings.
Applications-
- Used in making advanced electronic devices.
- Helps develop high-efficiency solar panels.
9. The Reverse Process- Deposition (Desublimation)
Concept-
- Deposition (or desublimation) is the reverse of sublimation, where a gas changes directly into a solid without forming a liquid.
Example-
- Frost formation on windows in winter when water vapor directly turns into ice.
Applications-
- Used in meteorology to study weather patterns.
- Plays a role in semiconductor fabrication.
Quiz on Sublimation- Test Your Understanding!
Here’s a mix of multiple-choice, true/false and open-ended questions to test students’ grasp of sublimation concepts.
Multiple-Choice Questions (MCQs)
1. Which of the following best describes sublimation?
a) A solid changing into a liquid
b) A solid changing directly into a gas
c) A liquid changing into a gas
d) A gas changing into a solid
2. Which of these substances undergoes sublimation at room temperature?
a) Water
b) Salt
c) Naphthalene balls
d) Sugar
3. What happens to the molecules of a solid during sublimation?
a) They gain enough energy to transition directly into gas form.
b) They lose energy and become a liquid first.
c) They become smaller and break down into new elements.
d) They move slower and turn into a liquid.
4. In a phase diagram, sublimation occurs when-
a) Pressure is very high
b) Temperature is very low
c) Pressure is very low
d) The substance is at its triple point
5. What is the name of the process opposite to sublimation (gas turning into solid)?
a) Deposition
b) Condensation
c) Freezing
d) Evaporation
True or False Questions
6. Sublimation occurs when a substance skips the liquid phase and directly turns into gas. (True / False)
7. Dry ice sublimates at room temperature because carbon dioxide cannot exist as a liquid under normal atmospheric pressure. (True / False)
8. Water can undergo sublimation under normal conditions. (True / False)
9. Sublimation only occurs in artificial or man-made conditions. (True / False)
10. Sublimation is useful in space science, food preservation and industrial purification. (True / False)
Open-Ended Questions (Short Answer & Discussion)
11. Explain why dry ice is commonly used in fog effects instead of normal ice.
12. Give two real-world applications of sublimation in industry or daily life.
13. How does sublimation play a role in preserving food using freeze-drying?
14. What happens to a piece of naphthalene (mothball) kept in a closed box for a few days? Why?
15. Describe how sublimation contributes to the formation of a comet’s tail when it approaches the Sun.
Bonus Challenge- Experiment Design
16. Design a simple experiment to demonstrate sublimation at home or in a school lab. Include the materials needed, procedure and observations.
Answer Key (for Teachers or Self-Check)
- b) A solid changing directly into a gas
- c) Naphthalene balls
- a) They gain enough energy to transition directly into gas form
- c) Pressure is very low
- a) Deposition
- True
- True
- False (Water can sublimate under very specific conditions, such as low pressure, but not normally)
- False (It occurs naturally, e.g., in snow and glaciers)
- True