Table of Contents
What Is a Solution?
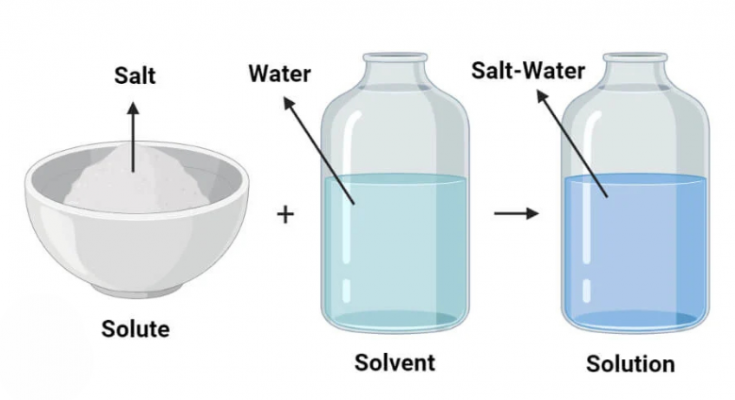
A solution is a homogeneous mixture formed when one or more substances (solutes) are evenly dispersed in another substance (the solvent). The particles of the solute are so small that they cannot be seen with the naked eye and do not settle out over time. This even distribution creates a single-phase system, meaning the solution looks the same throughout and behaves as a single substance, even though it contains multiple components.
Key Characteristics of a Solution
- Uniform composition– The solute is distributed at the molecular or ionic level.
- Stable mixture– The solute does not settle over time or separate upon standing.
- Cannot be filtered easily– The dissolved particles are too small to be separated by filtration.
Examples-
- Saltwater– Salt (solute) is dissolved in water (solvent).
- Soda– Carbon dioxide gas (solute) is dissolved in water (solvent) under pressure.
- Alloys– Brass is a solid solution where zinc (solute) is dissolved in copper (solvent).
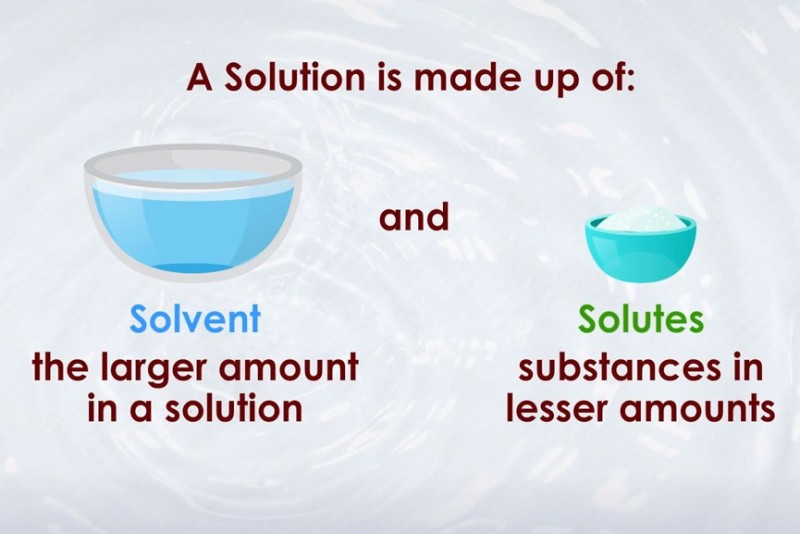
What Is a Solute?
A solute is the substance that is dissolved in a solution. It can exist in a solid, liquid, or gaseous state before dissolving. Typically, the solute is present in a smaller amount compared to the solvent. The nature of the solute—its molecular structure, size, surface area, polarity and ionic character—affects how easily it dissolves and how it behaves in the solution.
How Solutes Behave-
- Solute particles are broken apart by the solvent molecules.
- These particles are then surrounded by solvent molecules, a process called solvation (or hydration when the solvent is water).
- Solutes may dissociate into ions (e.g., NaCl in water) or remain as molecules (e.g., sugar in water).
Forms of Solutes-
- Solid– Table salt, sugar
- Liquid– Alcohol in water
- Gas– Carbon dioxide in soft drinks
Also Check – What are the Characteristics of the Particles of Matter ?
What Is a Solvent?
A solvent is the component of a solution that is present in the greatest amount and serves as the medium in which the solute dissolves. Solvents are most commonly liquids, though they can also be gases or solids. The solvent determines the physical state of the solution and often influences the solution’s chemical properties.
Functions of a Solvent-
- Disperses solute particles evenly throughout the mixture.
- Controls the physical state of the solution (e.g., if water is used, the solution is liquid).
- Interacts chemically or physically with the solute, often using molecular attractions (e.g., dipole interactions, hydrogen bonds).
Types of Solvents Based on Composition-
a) Polar Solvents-
- Have molecules with positive and negative poles.
- Good at dissolving ionic or other polar substances.
- Example– Water, ethanol.
b) Nonpolar Solvents-
- Molecules have balanced charge distribution.
- Best for dissolving nonpolar substances like oils and fats.
- Example– Benzene, hexane.
Difference between Solute and Solvent
| Feature | Solute | Solvent |
| Definition | The substance dissolved in a solution | The substance that dissolves the solute |
| Quantity | Present in smaller amount | Present in larger amount |
| Physical State | Solid, liquid, or gas | Usually a liquid (can be gas or solid) |
| Role in Solution | Becomes uniformly distributed | Acts as the medium for dispersion |
| Boiling Point | Generally higher | Generally lower |
| Determines Solubility | Depends on its molecular properties | Depends on polarity, temperature and bonding |
| Phase Behavior | Does not determine the final phase | Dictates the phase of the overall solution |
| Examples | Salt, sugar, CO₂, oxygen, ethanol | Water, alcohol, acetone, toluene, hexane |
Types of Solutions (Based on Physical State)
| Type of Solution | Solute State | Solvent State | Example |
| Gas in Gas | Gas | Gas | Air (oxygen in nitrogen) |
| Gas in Liquid | Gas | Liquid | Carbon dioxide in soda |
| Liquid in Liquid | Liquid | Liquid | Alcohol in water |
| Solid in Liquid | Solid | Liquid | Sugar in tea |
| Solid in Solid | Solid | Solid | Brass (zinc in copper) |
Concepts Related to Solubility
Solubility and saturation levels are core ideas in chemistry that explain how substances interact in a solution. These concepts are especially important in real-world applications such as cooking, medicine, environmental science and industrial chemistry.
Solubility
Definition–
Solubility is the maximum amount of a solute that can be dissolved in a fixed amount of solvent at a specific temperature and pressure, resulting in a stable solution.
Key Points-
- Expressed in units like grams per 100 mL or moles per liter.
- Every substance has a solubility limit.
- Once this limit is reached, the solution becomes saturated.
Example-
At 25°C, about 36 grams of salt (NaCl) can dissolve in 100 mL of water. Adding more salt won’t dissolve—it will settle at the bottom.
Factors Affecting Solubility
Understanding what affects solubility helps in controlling how much of a solute will dissolve.
1. Temperature-
- For most solids, solubility increases with temperature.
- Example– Sugar dissolves faster and in greater amounts in hot tea than in cold water.
- Example– Sugar dissolves faster and in greater amounts in hot tea than in cold water.
- For gases, solubility decreases as temperature increases.
- Example– Soda goes flat faster at warm temperatures because carbon dioxide escapes more easily.
- Example– Soda goes flat faster at warm temperatures because carbon dioxide escapes more easily.
2. Pressure (mainly for gases)-
- Increasing pressure increases the solubility of gases in liquids.
- Example– Soda is bottled under high pressure to keep CO₂ dissolved. When opened, pressure drops and the gas escapes (fizzing).
- Example– Soda is bottled under high pressure to keep CO₂ dissolved. When opened, pressure drops and the gas escapes (fizzing).
- Henry’s Law describes this relationship- the amount of gas dissolved is proportional to the pressure above the liquid.
3. Surface Area of Solute-
- Smaller or powdered particles dissolve faster because they have more surface area exposed to the solvent.
- Example– Crushed sugar cubes dissolve faster than whole cubes in water.
- Example– Crushed sugar cubes dissolve faster than whole cubes in water.
4. Nature of Solute and Solvent-
- Polarity matters- “Like dissolves like”.
- Polar solutes dissolve in polar solvents.
- Non-polar solutes dissolve in non-polar solvents.
- Example– Salt (polar) dissolves in water (polar), but not in oil (non-polar).
- Polar solutes dissolve in polar solvents.
Also Check – Properties of Matter: Physical, Chemical, and Functional Explained With Examples
Saturation Levels
Saturation levels describe how much solute is currently dissolved in a solvent, relative to the solvent’s capacity.
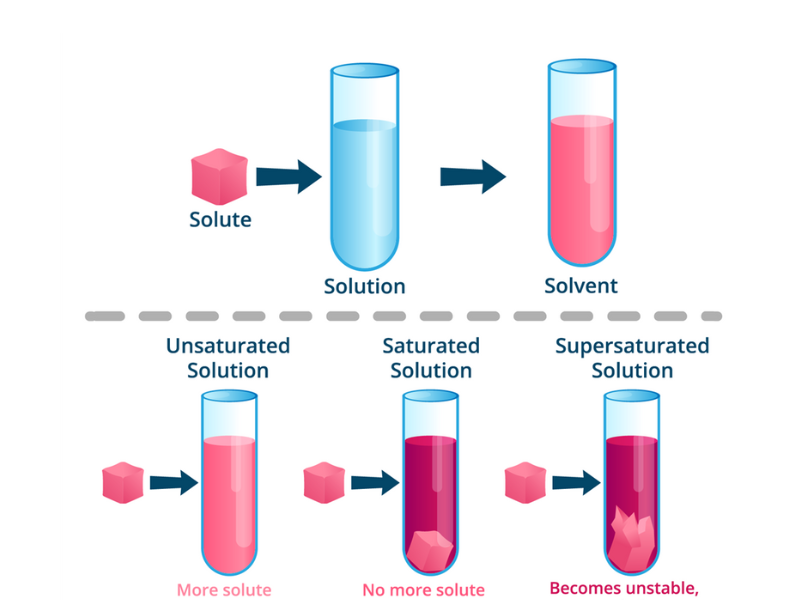
1. Unsaturated Solution-
- Contains less solute than the maximum it can hold at that temperature.
- More solute can still dissolve.
- Appears completely clear and stable.
Example– A glass of water with only a small spoon of sugar; more sugar can be added and will still dissolve.
2. Saturated Solution-
- Holds the maximum amount of solute that can dissolve at a specific temperature and pressure.
- Any added solute will not dissolve and will remain visible as solid particles.
- This is the solubility limit in action.
Example– Stirring sugar into water until no more dissolves and you see sugar settling at the bottom.
3. Supersaturated Solution-
- Contains more solute than normally possible at that temperature.
- Created by dissolving solute at higher temperatures, then cooling the solution slowly without disturbing it.
- Unstable– slight agitation or adding a crystal causes rapid crystallization.
Example– Making rock candy—by heating water to dissolve more sugar than usual and then cooling it to grow crystals.
Why It Matters-
- Cooking– Knowing when a sugar solution is saturated helps in candy making.
- Medicine– Solubility affects how drugs dissolve and get absorbed.
- Environment– Gas solubility in water affects aquatic life (oxygen availability).
- Industrial– Solubility affects product formulations like paint, detergents and beverages.
Categories of Solvents by Chemical Composition
Solvents are substances used to dissolve solutes, forming solutions. Based on their chemical makeup, solvents are grouped into three major categories–
A) Hydrocarbon Solvents
1. Chemical Composition-
Hydrocarbon solvents consist only of carbon (C) and hydrogen (H) atoms. They are non-polar molecules, meaning they do not have positive or negative poles in their molecular structure. This makes them suitable for dissolving other non-polar substances like oils, greases and waxes.
2. Origin-
They are derived from petroleum refining—specifically, from the volatile fractions obtained during the distillation of crude oil. These solvents can vary based on their carbon chain length and molecular structure.
3. Types of Hydrocarbon Solvents-
- Aliphatic hydrocarbons– Straight or branched chains (e.g., hexane).
- Aromatic hydrocarbons– Contain benzene rings (e.g., toluene, xylene).
- Naphthenic hydrocarbons– Saturated cyclic compounds (less common).
4. Characteristics-
- Low polarity– Best for dissolving non-polar compounds.
- Flammable– Require careful storage and handling.
- Volatile– Evaporate quickly at room temperature.
- Not water-miscible– They do not mix with water.
5. Uses-
- Industrial degreasers
- Adhesives and sealants
- Paint thinners
- Fuels (e.g., gasoline is a hydrocarbon mixture)
6. Examples-
- Hexane– Used for oil extraction and cleaning.
- Toluene– Common in paints, glues and industrial cleaning.
- Mineral spirits– Used in dry cleaning and painting.
B) Oxygenated Solvents
1. Chemical Composition-
Oxygenated solvents contain atoms of carbon, hydrogen and oxygen. They are polar solvents, meaning they have regions of positive and negative charge, which makes them capable of dissolving both polar and some non-polar substances.
2. Functional Groups-
These solvents include various functional groups that involve oxygen-
- Alcohols (–OH)- e.g., ethanol, methanol
- Ketones (C=O)- e.g., acetone
- Esters (COO)- e.g., ethyl acetate
- Ethers and glycols
3. Characteristics-
- High solvency power– Can dissolve a wide range of substances.
- Lower toxicity compared to halogenated solvents.
- Water-miscible (many are) and biodegradable (some types).
- Mild odor and low volatility in some types.
4. Uses-
- Paints and varnishes– Fast evaporation helps quick drying.
- Cosmetics and perfumes– Non-toxic and skin-safe solvents.
- Pharmaceuticals– As carriers or reactants in drug synthesis.
- Cleaning agents– For electronics, lenses and surfaces.
5. Examples-
- Acetone– Common nail polish remover and cleaner.
- Methanol– Used in fuels and formaldehyde production.
- Ethyl acetate– Found in glues and perfumes.
C) Halogenated Solvents
1. Chemical Composition-
These solvents contain one or more halogen atoms—chlorine (Cl), bromine (Br), iodine (I), or fluorine (F)—in addition to carbon and hydrogen. The inclusion of halogens makes these solvents highly polar and chemically reactive.
2. Characteristics-
- Extremely effective solvents for oils, waxes, greases and synthetic polymers.
- Often non-flammable, making them safer in high-temperature applications.
- Toxic and persistent in the environment—many are non-biodegradable.
- Can be carcinogenic or harmful with prolonged exposure.
- Require special disposal methods due to environmental hazards.
3. Uses-
- Dry cleaning– Particularly perchloroethylene.
- Degreasing metals in industrial settings.
- Electronics cleaning and precision instruments.
- Pharmaceutical and chemical manufacturing.
4. Examples-
- Perchloroethylene (PCE)– Primary dry cleaning solvent.
- Chloroform– Historical anesthetic and now a lab reagent.
- Trichloroethylene (TCE)– Used in degreasing and refrigerant production.
Comparison Table
| Category | Elements Involved | Polarity | Common Uses | Examples |
| Hydrocarbon Solvents | Carbon & Hydrogen | Non-polar | Fuels, degreasers, adhesives | Hexane, toluene |
| Oxygenated Solvents | Carbon, Hydrogen, Oxygen | Polar | Paints, perfumes, pharmaceuticals | Acetone, ethyl acetate |
| Halogenated Solvents | C, H, + Halogen (Cl, Br) | Strongly polar | Dry cleaning, industrial cleaning | Perchloroethylene, chloroform |
Difference Between Solute, Solvent and Solution
| Feature | Solute | Solvent | Solution |
| Definition | A substance that is dissolved in a solvent. | A substance that dissolves the solute. | A homogeneous mixture of solute and solvent. |
| Role | Dispersed or broken down into particles. | Acts as the medium in which solute dissolves. | The final, uniform mixture formed after solute dissolves in solvent. |
| Amount in Mixture | Present in a smaller amount. | Present in a greater amount. | Combines both solute and solvent in defined proportions. |
| Phase | Can be solid, liquid, or gas. | Typically a liquid, but can also be gas or solid. | The phase of the solution usually matches that of the solvent. |
| Visibility | Not visible once dissolved. | Visible only before mixing. | Appears as a single-phase (uniform) mixture. |
| Effect on Boiling Point | Typically raises the boiling point of the solvent (boiling point elevation). | Original boiling point usually lower than the solution. | Boiling point is affected by the nature and amount of solute. |
| Effect on Freezing Point | Lowers the freezing point of the solvent (freezing point depression). | Has a higher freezing point before solute is added. | The solution freezes at a lower temperature than the pure solvent. |
| Determines | Affects the concentration and properties of the solution. | Determines the state and solvency capability. | Determined by both the solute and solvent interacting. |
| Chemical Nature | Can be ionic (like NaCl) or molecular (like sugar). | Often polar or non-polar depending on solubility needs. | May be polar or non-polar depending on the combination used. |
| Intermolecular Interaction | Interacts with solvent through ion-dipole or hydrogen bonds. | Surrounds solute molecules, enabling them to disperse. | Result of molecular interactions between solute and solvent. |
| Solubility Dependent On | Nature of solute, particle size, temperature, pressure. | Polarity, temperature and ability to interact with the solute. | Solubility characteristics of both solute and solvent define the solution. |
| Examples | Salt, sugar, oxygen, ethanol, carbon dioxide. | Water, alcohol, acetone, benzene. | Saltwater, sugar water, soda, air, brass (solid solution). |
Also Check – Differentiate between Homogeneous mixture and Heterogeneous mixture
Example (Saltwater Solution)-
| Component | Role | Behavior |
| Salt | Solute | Dissolves into ions (Na⁺ and Cl⁻) in water |
| Water | Solvent | Surrounds and separates salt ions, allowing them to disperse |
| Saltwater | Solution | Appears uniform; salty taste shows the presence of salt uniformly spread |