Table of Contents
What Is Matter?
Matter is defined as any substance that has both mass and occupies space. All physical objects are composed of matter, regardless of their size or state. In classical physics and chemistry, matter is characterized by its physical and chemical properties, which determine how it behaves under different conditions and in interactions with other forms of matter and energy.
How Are Properties of Matter Classified?
The properties of matter can be systematically classified into two broad categories:
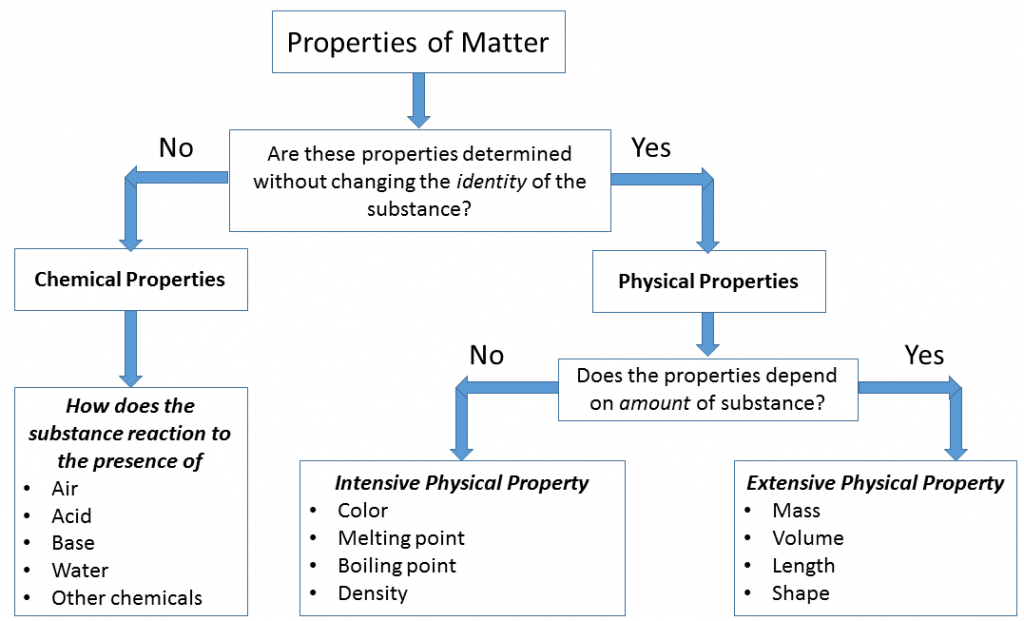
Physical Properties
Physical properties are characteristics of a substance that can be observed or measured without changing its chemical composition. These include aspects like state, color, density, melting point, and boiling point.
Chemical Properties
Chemical properties are observed only during a chemical reaction, and they involve a change in the substance’s chemical identity. They describe the potential of a substance to undergo specific chemical changes.
Each of these categories can be further divided based on whether or not the property depends on the amount of material present.
What Are the Physical Properties of Matter
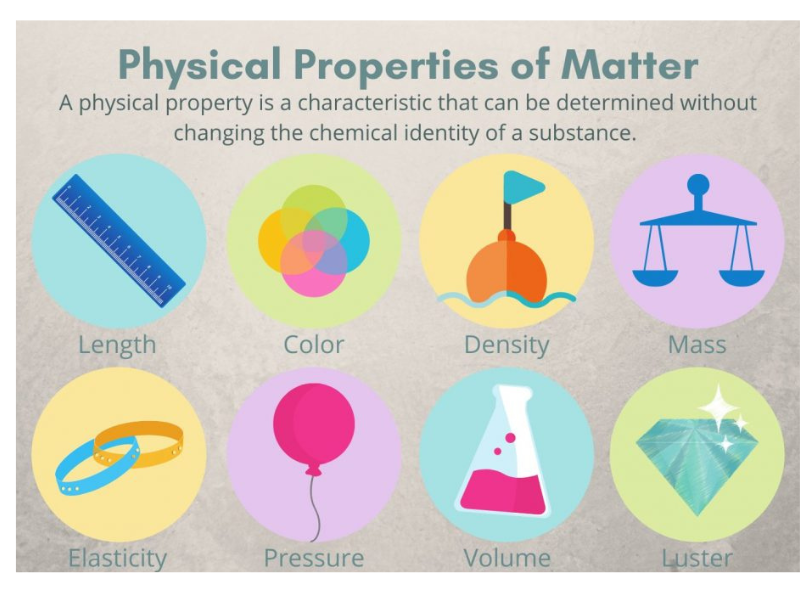
What Are Extensive Physical Properties?
These depend on the quantity of matter present. They are not inherent to the substance and change with the system size.
- Mass: A measure of the amount of matter in an object, typically in kilograms or grams. It is a scalar quantity and remains constant regardless of location.
- Volume: The amount of three-dimensional space occupied by a substance. Common units include liters (L), cubic meters (m³), or cubic centimeters (cm³).
- Length/Dimensions: Linear extent of matter (e.g., height, width, depth), often used in calculating other physical properties.
- Total Energy: The sum of all forms of energy (thermal, kinetic, potential) in a system. This is an extensive property because it scales with mass.

What Are Intensive Physical Properties?
These do not depend on the amount of material. They are intrinsic to the substance and are useful for identification and classification.
- Density (ρ): Defined as mass per unit volume (ρ = m/V). For example, water has a density of 1.0 g/cm³ at 4°C.
- Melting Point: The temperature at which a solid becomes a liquid. It is a fixed point for pure substances.
- Boiling Point: The temperature at which a liquid’s vapor pressure equals atmospheric pressure, causing it to change into a gas.
- Refractive Index: A measure of how much light bends, or refracts, when entering a material.
- Thermal and Electrical Conductivity: The ability of a material to conduct heat or electricity, respectively.
- Color and Luster: Optical properties related to how a substance interacts with visible light.
- Hardness: Resistance to scratching or indentation, often measured by Mohs scale.
- Malleability: The capacity of a substance to deform under compressive stress (e.g., forming sheets).
- Ductility: The capacity to be drawn into wires without breaking.
- Solubility: The ability of a substance to dissolve in a solvent (e.g., sugar in water).
- State of Matter: Whether the substance exists as a solid, liquid, gas, plasma, or Bose-Einstein condensate under standard conditions.

What Are the Chemical Properties of Matter?
Chemical Properties of Matter
Chemical properties involve the ability of a substance to undergo chemical transformations that result in the formation of new substances.
Common chemical properties include:
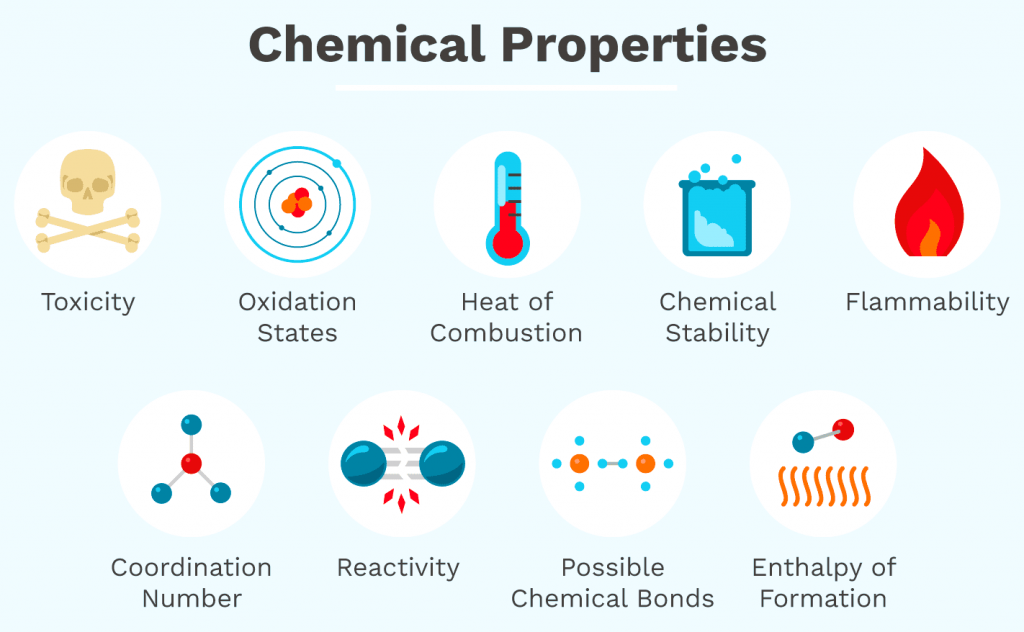
- Flammability: The ability to combust or burn in the presence of oxygen, forming new chemical products like CO₂ and H₂O.
- Reactivity: How readily a substance engages in chemical reactions with other substances, including acids, bases, water, or oxygen.
- Toxicity: The degree to which a chemical can damage living organisms.
- Oxidation State: The effective charge on an atom in a compound, which influences redox reactions.
- pH Value: A measure of the hydrogen ion concentration in a solution, indicating acidity (<7), neutrality (7), or basicity (>7).
- Heat of Combustion: The amount of energy released as heat when a substance undergoes complete combustion with oxygen.
- Chemical Stability: Resistance of a substance to change under specified conditions.
- Corrosiveness: The ability to deteriorate materials, often metals, through chemical reactions (e.g., rusting of iron).
- Radioactivity: The spontaneous decay of atomic nuclei, emitting particles and radiation.
Note: Unlike physical properties, these are only observable through experimental chemical changes
Also Check – What are the Characteristics of the Particles of Matter ?
What Are the States of Matter and Their Properties?
Matter can exist in several physical states. Each has its own structure and behavior.
| State | Shape | Volume | Compressibility | Particles Are |
| Solid | Fixed | Fixed | Not easily compressible | Tightly packed and ordered |
| Liquid | Takes container shape | Fixed | Slightly compressible | Loosely packed, slide around |
| Gas | Takes container shape | Expands to fill space | Easily compressible | Far apart, move freely |
| Plasma | No fixed shape | No fixed volume | Very compressible | Ionized particles, electrically active |
| Bose-Einstein Condensate | Fixed | Fixed | Almost no movement | Atoms act as one, very low energy |
Additional Functional Properties of Matter
Beyond the basic properties, various scientific fields examine specialized properties for applied purposes:
- Viscosity: Resistance to flow; higher in substances like honey, lower in water.
- Surface Tension: Energy required to increase the surface area of a liquid; crucial in capillarity and fluid dynamics.
- Elasticity: A measure of how well a material returns to its original shape after deformation.
- Tensile Strength: Maximum stress that a material can withstand while being stretched or pulled.
- Thermal Expansion: Tendency of matter to change volume in response to temperature changes.
- Latent Heat: Energy absorbed or released during a phase change without temperature variation (e.g., melting or boiling).
- Specific Heat Capacity: Amount of heat required to raise the temperature of 1 gram of substance by 1°C.
- Permeability: Ability of a material to allow substances (gases or liquids) to pass through.
- Crystallinity: Degree of structural order in a solid; affects mechanical, thermal, and optical properties.
- Magnetism: The property that allows materials to attract or repel under a magnetic field, commonly observed in ferromagnetic materials.
FAQs
Conceptual Question & Answer
1. What is the difference between physical and chemical properties of matter? Give one example of each.
Answer:
Physical properties can be observed or measured without changing the substance’s chemical identity, such as melting point or density.
Chemical properties describe a substance’s ability to undergo a chemical change, such as flammability or reactivity with acid.
Example: Melting point (physical), ability to rust (chemical).
2. Why does the mass of a substance remain constant even when its shape changes?
Answer:
Mass remains constant because it represents the quantity of matter, which is not affected by changes in shape. This is an application of the law of conservation of mass, which states that matter cannot be created or destroyed in physical processes.
3. Why does water expand when it freezes, unlike most substances?
Answer:
Water molecules form a structured hexagonal lattice due to hydrogen bonding when frozen. This arrangement increases the volume, making ice less dense than liquid water.
4. Why can gases be compressed more easily than solids or liquids?
Answer:
Gas particles are spaced far apart and have significant empty space between them. When pressure is applied, they can be pushed closer together, unlike in solids and liquids where particles are already tightly packed.
5. How can you explain diffusion in gases using the kinetic molecular theory?
Answer:
According to the kinetic molecular theory, gas particles move in constant, random motion. Because of this motion and the large spaces between particles, they naturally spread out to fill a container, resulting in diffusion.
6. A student heats a metal rod and finds it expands. What does this suggest about the particles in the rod?
Answer:
Heating provides energy to the particles, increasing their vibration. This causes them to move slightly further apart, leading to expansion. This supports the particle model of matter, which states that particles in a solid vibrate but remain in fixed positions.
7. How does density help identify a substance?
Answer:
Density is a characteristic property of a pure substance. If a material’s mass and volume are measured, its density can be calculated and compared to known values to help identify the material.
8. Why does ice float on water even though both are made of H₂O?
Answer:
Ice is less dense than water due to the open, crystalline structure formed during freezing. This structure occupies more volume, lowering the density and causing ice to float.
9. Why do solids have a fixed shape but gases do not?
Answer:
In solids, particles are closely packed and held together by strong intermolecular forces, allowing only vibrations in place. In gases, particles are far apart and move freely, enabling them to expand and take the shape of their container.
10. Why does increasing temperature usually decrease the density of a substance?
Answer:
When temperature increases, particles gain kinetic energy and move further apart, increasing the volume. Since density equals mass divided by volume, an increase in volume results in lower density.
11. How do intermolecular forces affect the state of matter?
Answer:
Stronger intermolecular forces hold particles tightly together in solids. In liquids, the forces are moderate, allowing particles to move past each other. In gases, forces are weak or negligible, and particles move independently.
12. A gas is compressed in a cylinder. What happens to its temperature if no heat escapes?
Answer:
If compression occurs without heat loss (an adiabatic process), the temperature increases. This is because the work done on the gas increases the kinetic energy of the particles, raising the temperature.
13. Why is boiling point a better indicator of purity than melting point?
Answer:
Boiling points are more sensitive to impurities than melting points. Even small amounts of impurities can cause significant deviations in the boiling point, making it a more reliable test for purity.
14. What’s the difference between a mixture and a compound, based on their properties?
Answer:
In a mixture, components retain their original properties and can be separated physically. In a compound, elements combine chemically and form new properties different from the original elements. Compounds can only be separated by chemical means.
15. You have two colorless liquids. One has a constant boiling point, the other does not. What does that tell you?
Answer:
A constant boiling point indicates a pure substance. A variable or changing boiling point suggests a mixture, since the components may evaporate at different temperatures.
MCQs- Properties of Matter (Conceptual)
1. Which of the following is a chemical property of matter?
A. Boiling point
B. Density
C. Reactivity with acid
D. Color
Answer: C. Reactivity with acid
Explanation: Chemical properties describe how a substance reacts; reactivity is one such property.
2. Which state of matter has a definite volume but no definite shape?
A. Solid
B. Liquid
C. Gas
D. Plasma
Answer: B. Liquid
Explanation: Liquids take the shape of their container but have a fixed volume.
3. When a solid turns directly into a gas without becoming a liquid, the process is called:
A. Evaporation
B. Condensation
C. Sublimation
D. Melting
Answer: C. Sublimation
Explanation: Sublimation is the direct phase transition from solid to gas.
4. What happens to the particles of a substance when it is heated?
A. They slow down
B. They stop moving
C. They get closer together
D. They move faster and further apart
Answer: D. They move faster and further apart
Explanation: Heat increases kinetic energy, causing particles to move more rapidly.
5. Which of the following is not a physical property?
A. Melting point
B. Flammability
C. Solubility
D. Density
Answer: B. Flammability
Explanation: Flammability involves a chemical reaction, making it a chemical property.
6. A substance has a fixed shape and volume. It is most likely a:
A. Gas
B. Liquid
C. Solid
D. Plasma
Answer: C. Solid
Explanation: Solids have both definite shape and volume due to tightly packed particles.
7. Which of the following best explains why ice floats on water?
A. Ice is heavier than water
B. Ice molecules are less polar
C. Ice has a lower density due to its structure
D. Ice molecules are more strongly bonded
Answer: C. Ice has a lower density due to its structure
Explanation: Hydrogen bonding creates an open crystalline structure in ice, reducing its density.
8. The ability of a substance to be hammered into thin sheets is known as:
A. Brittleness
B. Ductility
C. Malleability
D. Elasticity
Answer: C. Malleability
Explanation: Malleability is the ability to deform under compressive stress without breaking.
9. Which process best describes gas particles spreading out evenly in a room?
A. Condensation
B. Diffusion
C. Evaporation
D. Deposition
Answer: B. Diffusion
Explanation: Diffusion occurs as gas particles move from areas of high to low concentration.
10. During boiling, which of the following remains constant?
A. Temperature
B. Volume
C. Pressure
D. Mass
Answer: A. Temperature
Explanation: During a phase change like boiling, temperature remains constant until all the substance has changed state.
Experiment-Based Reasoning Questions- Properties of Matter
1. A student heats a beaker of water and records the temperature every minute. The temperature increases steadily until it reaches 100°C, where it stops rising for several minutes despite continuous heating. What does this indicate?
Answer:
This indicates that a phase change (boiling) is occurring at 100°C. The energy is being used to break intermolecular bonds, not to increase temperature. This demonstrates the latent heat of vaporization.
2. A teacher places a few drops of food coloring in two beakers—one with hot water, the other with cold water. The color spreads faster in hot water. Why?
Answer:
In hot water, particles move faster due to higher kinetic energy, which increases the rate of diffusion. This demonstrates how temperature affects particle motion.
3. A metal cube and a wooden cube of the same size are placed in water. The metal sinks while the wood floats. What conclusion can be drawn from this observation?
Answer:
The metal cube has a higher density than water, causing it to sink. The wood’s density is lower than water, so it floats. This illustrates density as a factor in buoyancy.
4. A student compresses a sealed syringe filled with air and observes that the plunger moves back when released. What property of gases does this demonstrate?
Answer:
It demonstrates that gases are compressible and exert pressure. When compressed, gas particles are forced closer together; releasing the plunger lets them expand, pushing it back due to restoring pressure.
5. In an experiment, a substance is heated and begins to melt at a fixed temperature without changing for several minutes. What does this constant temperature during melting tell us?
Answer:
It shows the melting point of a pure substance. The energy input is used to overcome interparticle forces, not to increase temperature, which reflects latent heat of fusion.
6. Two transparent liquids are mixed. A white solid forms and settles at the bottom. What type of change is this, and how do you know?
Answer:
This is a chemical change, evidenced by the formation of a precipitate. A new substance is formed, which indicates a chemical reaction between the components.
7. In an experiment, a gas is collected over water. The collected gas is passed through limewater, turning it milky. What is the gas and what does this indicate?
Answer:
The gas is carbon dioxide (CO₂). Its reaction with limewater (calcium hydroxide) to form calcium carbonate demonstrates a chemical change and gas identification technique.
8. A student places three candles under jars of different sizes and measures how long each candle burns. The smallest jar’s candle goes out the fastest. What does this tell us about gases?
Answer:
It shows that oxygen is needed for combustion, and the limited air in the smaller jar gets used up faster. This demonstrates that gases occupy space and play a role in supporting chemical reactions.
9. A student puts salt in water and stirs. The salt disappears. What physical property is being demonstrated, and how could the salt be recovered?
Answer:
This demonstrates solubility, a physical property. The salt can be recovered by evaporating the water, showing that no new substance was formed, so it’s a physical change.
10. A metal wire is bent and then released. It returns to its original shape. What property does this experiment demonstrate?Answer:
It demonstrates elasticity, the ability of a material to return to its original shape after deformation. This is a physical property of matter.

