Table of Contents
Chapter – 1 – Matter in Our Surroundings Concepts Included in the Chapter-
- What is Matter?
An exploration of how everything around us is made of matter, including solids, liquids, and gases. Understanding the physical properties and characteristics of matter, including mass and volume. - Physical Nature of Matter-
A look at how matter is composed of tiny particles, how these particles move and interact, and how different states of matter behave. - States of Matter-
Understanding the three states of matter- solid, liquid, and gas, and their key differences in terms of particle arrangement, movement, and energy. - Changes in State-
Explanation of how matter changes from one state to another through processes like melting, boiling, and sublimation, driven by changes in temperature and pressure. - Evaporation and its Effects-
A look at evaporation as a surface phenomenon, how it cools down the surroundings, and the factors that affect its rate, such as temperature, surface area, humidity, and wind speed. - Latent Heat-
An introduction to the concepts of latent heat of fusion and vaporization, explaining the energy required for substances to change states without changing temperature. - Factors Affecting the State of Matter-
Understanding how temperature and pressure influence the state of matter, and how substances like carbon dioxide can sublimate or deposit.
NCERT Exemplar Class 9 Science Chapter – 1 – Matter in Our Surroundings- (Solved with detailed explanation)
Multiple Choice Questions
1. Which one of the following sets of phenomena would increase on raising the temperature?
Options-
(a) Diffusion, evaporation, compression of gases
(b) Evaporation, compression of gases, solubility
(c) Evaporation, diffusion, expansion of gases
(d) Evaporation, solubility, diffusion, compression of gases
Correct Answer- (c) Evaporation, diffusion, expansion of gases
Explanation-
- When temperature increases, the kinetic energy of particles also increases, leading to faster diffusion (movement of particles from a higher to lower concentration).
- Evaporation also increases with temperature as more molecules gain enough energy to escape from the liquid phase to the gaseous phase.
- Expansion of gases occurs because increasing temperature makes gas molecules move more rapidly, causing them to occupy more space.
- Compression of gases would not increase with temperature; instead, gases tend to expand.
Also Check – NCERT Solutions for Class 9 Science Chapter 1- Matter In Our Surroundings
2. Seema visited a Natural Gas Compressing Unit and found that the gas can be liquefied under specific conditions of temperature and pressure. While sharing her experience with friends she got confused. Help her to identify the correct set of conditions.
Options-
(a) Low temperature, low pressure
(b) High temperature, low pressure
(c) Low temperature, high pressure
(d) High temperature, high pressure
Correct Answer- (c) Low temperature, high pressure
Explanation-
- To liquefy a gas, we need to bring the gas molecules closer together.
- High pressure forces the gas molecules to move closer to each other, reducing the intermolecular distance.
- Low temperature decreases the kinetic energy of gas molecules, reducing their tendency to move apart.
- Thus, low temperature and high pressure together help in the liquefaction of gases.
Also Check – Chapter 1 -MATTER IN OUR SURROUNDINGS -Class 9 – Simplified notes
3. The property to flow is unique to fluids. Which one of the following statements is correct?
Options-
(a) Only gases behave like fluids
(b) Gases and solids behave like fluids
(c) Gases and liquids behave like fluids
(d) Only liquids are fluids
Correct Answer- (c) Gases and liquids behave like fluids
Explanation-
- Fluids are substances that can flow and take the shape of the container they are placed in.
- Both liquids and gases can flow because their molecules are not held in a fixed position.
- In solids, particles are tightly packed, and they cannot flow.
- Hence, both gases and liquids are considered fluids.
4. During summer, water kept in an earthen pot becomes cool because of the phenomenon of-
Options-
(a) Diffusion
(b) Transpiration
(c) Osmosis
(d) Evaporation
Correct Answer- (d) Evaporation
Explanation-
- Earthen pots have tiny pores that allow water to slowly seep through and evaporate from the surface.
- Evaporation absorbs heat from the water inside the pot, making it cooler.
- This is the same principle that makes sweating an effective cooling mechanism for our body.
Also Check – Rapid Revision –Chapter 1- Matter In Our Surroundings – Class 9 Science
5. A few substances are arranged in the increasing order of ‘forces of attraction’ between their particles. Which one of the following represents a correct arrangement?
Options-
(a) Water, air, wind
(b) Air, sugar, oil
(c) Oxygen, water, sugar
(d) Salt, juice, air
Correct Answer- (c) Oxygen, water, sugar
Explanation-
- The strength of intermolecular forces increases in the order- gas < liquid < solid.
- Oxygen (a gas) has the weakest intermolecular forces.
- Water (a liquid) has moderate intermolecular forces.
- Sugar (a solid) has the strongest intermolecular forces.
- Thus, the correct order is Oxygen < Water < Sugar.
Also Check – 82 Reasoning-Based Questions & Answers-Matter In Our Surroundings -Class 9 Science
6. On converting 25°C, 38°C, and 66°C to the Kelvin scale, the correct sequence of temperatures will be-
Options-
(a) 298 K, 311 K, and 339 K
(b) 298 K, 300 K, and 338 K
(c) 273 K, 278 K, and 543 K
(d) 298 K, 310 K, and 338 K
Correct Answer- (a) 298 K, 311 K, and 339 K
Explanation-
- The Kelvin temperature is calculated by adding 273 to the Celsius value-
- 25°C + 273 = 298 K
- 38°C + 273 = 311 K
- 66°C + 273 = 339 K
7. Choose the correct statement from the following-
Options-
(a) Conversion of solid into vapours without passing through the liquid state is called sublimation.
(b) Conversion of vapours into solid without passing through the liquid state is called vaporisation.
(c) Conversion of vapours into solid without passing through the liquid state is called freezing.
(d) Conversion of solid into liquid is called sublimation.
Correct Answer- (a) Conversion of solid into vapours without passing through the liquid state is called sublimation.
Explanation-
- Sublimation is the process where a solid directly changes into gas without becoming liquid (e.g., camphor, dry ice).
- Freezing is the process of changing a liquid into solid.
- Vaporization is the process of changing a liquid into gas.
8. The boiling points of diethyl ether, acetone, and n-butyl alcohol are 35°C, 56°C, and 118°C respectively. Which one of the following correctly represents their boiling points on the Kelvin scale?
Options-
(a) 306 K, 329 K, 391 K
(b) 308 K, 329 K, 392 K
(c) 308 K, 329 K, 391 K
(d) 329 K, 392 K, 308 K
Correct Answer- (c) 308 K, 329 K, 391 K
Explanation-
- 35°C + 273 = 308 K
- 56°C + 273 = 329 K
- 118°C + 273 = 391 K
9. Which condition out of the following will increase the evaporation of water?
Options-
(a) Increase in temperature of water
(b) Decrease in temperature of water
(c) Less exposed surface area of water
(d) Adding common salt to water
Correct Answer- (a) Increase in temperature of water
Explanation-
- Higher temperature provides more kinetic energy to molecules, increasing evaporation.
- Increasing the surface area and reducing humidity also increase evaporation.
Also Check – Chapter 1- Matter In Our Surroundings – Class 9 Science – Long Question and Answers
10. In which of the following conditions, the distance between the molecules of hydrogen gas would increase?
(i) Increasing pressure on hydrogen contained in a closed container
(ii) Some hydrogen gas leaking out of the container
(iii) Increasing the volume of the container of hydrogen gas
(iv) Adding more hydrogen gas to the container without increasing the volume of the container
Options-
(a) (i) and (iii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Correct Answer- (c) (ii) and (iii)
Explanation-
- Leaking gas (ii) means fewer molecules in the container, increasing molecular distance.
- Increasing volume (iii) allows gas molecules to spread apart.
Short Answer Questions
11. A sample of water under study was found to boil at 102°C at normal temperature and pressure. Is the water pure? Will this water freeze at 0°C? Comment.
Answer-
- Pure water boils at 100°C at normal atmospheric pressure (1 atm). If the given sample boils at 102°C, it indicates the presence of impurities.
- Impurities increase the boiling point of water due to the phenomenon known as boiling point elevation.
- Similarly, the presence of impurities lowers the freezing point, meaning this water would freeze at a temperature below 0°C rather than exactly at 0°C.
- Therefore, this water sample is not pure.
12. A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following graphs would correctly represent the result? Justify your choice.
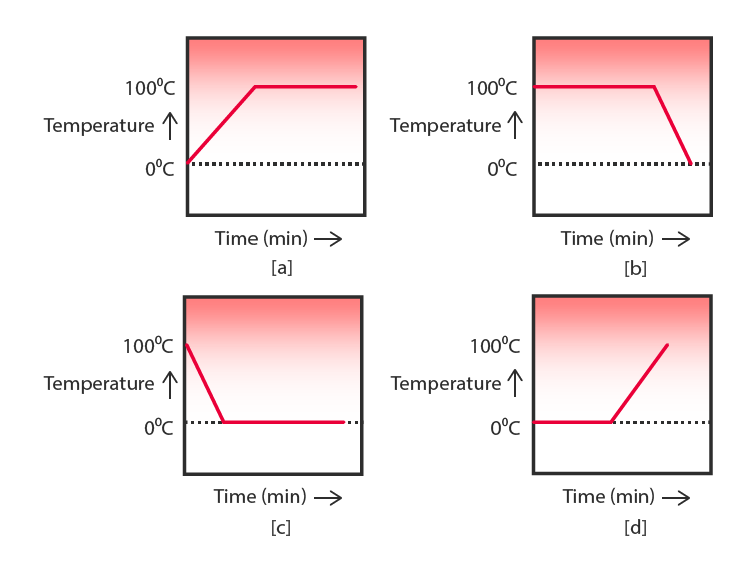
Answer-
- The correct graph should show a constant temperature initially at 0°C while the ice melts.
- Once the ice completely melts, the temperature starts increasing steadily as the water absorbs heat.
- This is because, during the melting process, all the heat supplied is used in overcoming intermolecular forces and converting ice into liquid water rather than increasing temperature.
- After complete melting, the temperature rises until boiling occurs at 100°C.
Also Check – Chapter 1- Matter In Our Surroundings – Class 9 Science – Solved MCQs
13. Fill in the blanks-
Answer-
(a) Evaporation of a liquid at room temperature leads to a cooling effect.
(b) At room temperature, the forces of attraction between the particles of solid substances are stronger than those which exist in the gaseous state.
(c) The arrangement of particles is less ordered in the liquid state. However, there is no order in the gaseous state.
(d) Sublimation is the change of solid state directly to vapour state without going through the liquid state.
(e) The phenomenon of change of a liquid into the gaseous state at any temperature below its boiling point is called evaporation.
Also Check – Sublimation- How Solids Turn Into Gas Without Melting
14. Match the physical quantities given in column A to their SI units given in column B.
Answer-
| (A) Physical Quantity | (B) SI Unit |
| (a) Pressure | (iii) Pascal (Pa) |
| (b) Temperature | (iv) Kelvin (K) |
| (c) Density | (v) Kilogram per cubic metre (kg/m³) |
| (d) Mass | (ii) Kilogram (kg) |
| (e) Volume | (i) Cubic metre (m³) |
15. The non-SI and SI units of some physical quantities are given in column A and column B respectively. Match the units belonging to the same physical quantity.
Answer-
| (A) Non-SI Unit | (B) SI Unit |
| (a) Degree Celsius | (iv) Kelvin (K) |
| (b) Centimetre | (iii) Metre (m) |
| (c) Gram per centimetre cube | (v) Kilogram per metre cube (kg/m³) |
| (d) Bar | (ii) Pascal (Pa) |
| (e) Milligram | (i) Kilogram (kg) |
16. ‘Osmosis is a special kind of diffusion’. Comment.
Answer-
- Diffusion is the movement of particles from a higher concentration to a lower concentration without any barrier.
- Osmosis, on the other hand, is a special type of diffusion that involves the movement of water molecules across a semipermeable membrane from a lower solute concentration to a higher solute concentration.
- Osmosis occurs in biological processes, such as water absorption by plant roots and water balance in human cells.
- Hence, osmosis is a type of diffusion, but it requires a semipermeable membrane.
Also Check – Solute, Solvent and Solution- Definition, Difference between, Characteristics, Examples
17. Classify the following into osmosis/diffusion-
| Process | Osmosis / Diffusion |
| (a) Swelling up of a raisin on keeping in water | Osmosis |
| (b) Spreading of virus on sneezing | Diffusion |
| (c) Earthworm dying on coming in contact with common salt | Osmosis |
| (d) Shrinking of grapes kept in thick sugar syrup | Osmosis |
| (e) Preserving pickles in salt | Osmosis |
| (f) Spreading of smell of cake being baked throughout the house | Diffusion |
| (g) Aquatic animals using oxygen dissolved in water during respiration | Diffusion |
18. Water as ice has a cooling effect, whereas water as steam may cause severe burns. Explain these observations.
Answer-
- Ice absorbs heat from its surroundings during melting, leading to a cooling effect.
- Steam carries extra energy in the form of latent heat of vaporization. When steam touches the skin, it releases this extra energy as it condenses into water.
- This sudden release of energy causes severe burns compared to boiling water at the same temperature.
19. Alka was making tea in a kettle. Suddenly she felt intense heat from the puff of steam gushing out of the spout of the kettle. She wondered whether the temperature of the steam was higher than that of the water boiling in the kettle. Comment.
Answer-
- The temperature of steam and boiling water is the same (100°C at 1 atm pressure).
- However, steam contains extra energy in the form of latent heat of vaporization.
- When steam condenses on the skin, it releases this latent heat, causing a stronger burning sensation than boiling water.
Also Check – Latent Heat of Fusion Formula- Easy Explanation with Formula & Examples
Also Check – What is Latent Heat? Fusion & Vaporization Explained with Real-Life Applications
20. A glass tumbler containing hot water is kept in the freezer compartment of a refrigerator (temperature < 0°C). If you could measure the temperature of the content of the tumbler, which of the following graphs would correctly represent the change in its temperature as a function of time?
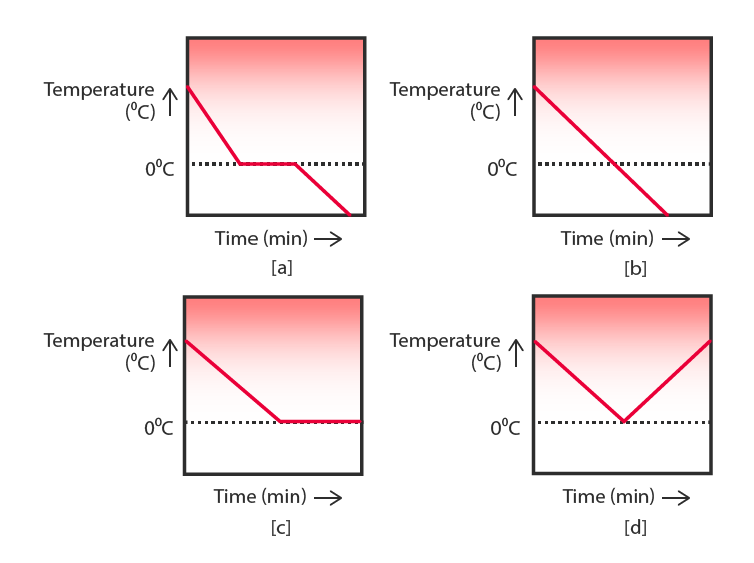
Answer-
- The correct graph should show-
- Gradual cooling of water till 0°C.
- Constant temperature at 0°C as the water freezes (latent heat is released).
- Further decrease in temperature after complete freezing.
21. Look at the diagram and suggest in which of the vessels A, B, C, or D the rate of evaporation will be the highest? Explain.
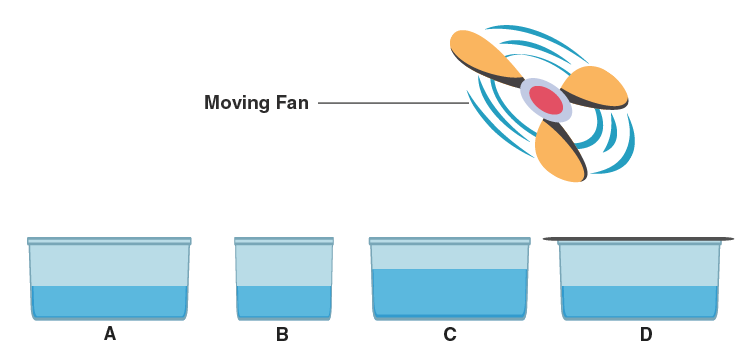
Answer-
- Evaporation is highest in vessel C because-
- It has the largest surface area, allowing more molecules to escape into the air.
- High wind speed helps remove water vapors quickly.
- Higher temperature increases kinetic energy, enhancing evaporation.
Also Check – The Science of Evaporation – Process, Factors, and Applications
22. (a) Conversion of solid to vapour is called sublimation. Name the term used to denote the conversion of vapour to solid.
Answer-
- The conversion of vapour directly into solid is called deposition.
- Example- Frost formation from water vapour in winter.
(b) Conversion of solid state to liquid state is called fusion; what is meant by latent heat of fusion?
Answer-
- Latent heat of fusion is the heat energy required to change a solid into a liquid at its melting point without changing its temperature.
- Example- Ice melts at 0°C but remains at 0°C until all the ice has turned into water.
Long Answer Questions
23. You are provided with a mixture of naphthalene and ammonium chloride by your teacher. Suggest an activity to separate them with a well-labelled diagram.
Answer-
Naphthalene and ammonium chloride can be separated using the sublimation method because both of these compounds sublimate directly from a solid to a gas without passing through a liquid phase.
Materials Required-
- Mixture of naphthalene and ammonium chloride
- China dish
- Inverted funnel
- Tripod stand
- Burner
- Cotton plug
Procedure-
- Take the mixture of naphthalene and ammonium chloride in a china dish.
- Place an inverted funnel over the china dish.
- Plug the narrow end of the funnel with cotton to prevent vapors from escaping.
- Heat the mixture gently with a burner.
- Ammonium chloride and naphthalene will sublimate, turning into vapours, and will condense on the cooler parts of the funnel.
- The condensed naphthalene and ammonium chloride crystals can be collected separately.
Conclusion-
By using sublimation, solid naphthalene and ammonium chloride can be effectively separated without using any solvents.
Diagram-
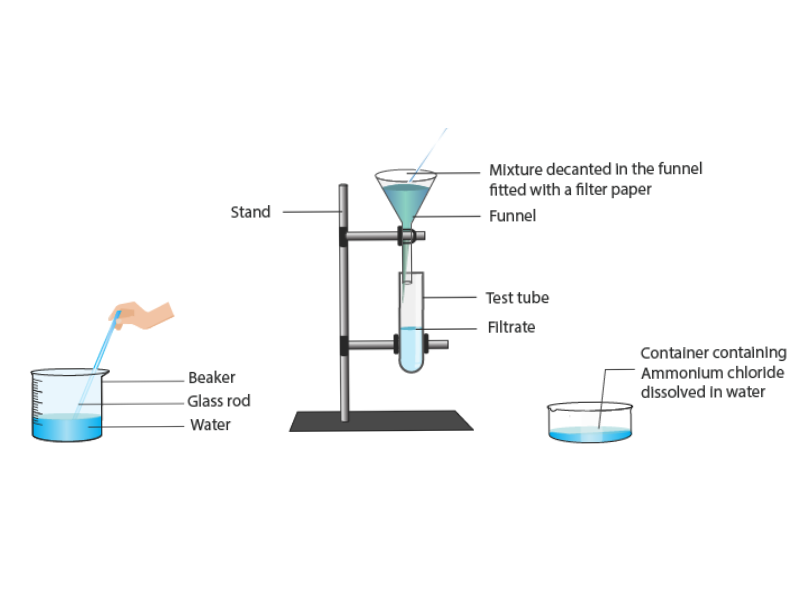
24. It is a hot summer day, Priyanshi and Ali are wearing cotton and nylon clothes respectively. Who do you think would be more comfortable and why?
Answer-
- Priyanshi (wearing cotton) would be more comfortable than Ali (wearing nylon).
- Cotton is a good absorber of sweat, whereas nylon is non-absorbent and traps heat.
- Cotton fibers allow better air circulation, helping sweat evaporate quickly, which cools the body.
- On the other hand, nylon does not absorb sweat, leading to discomfort and excessive heat retention.
- Conclusion- Priyanshi will feel more comfortable as cotton promotes cooling through evaporation.
25. You want to wear your favourite shirt to a party, but the problem is that it is still wet after a wash. What steps would you take to dry it faster?
Answer-
To dry the shirt faster, the following methods can be used-
- Increase air circulation-
- Hang the shirt in an area with good air movement or under a fan.
- Wind speeds up evaporation by removing humid air around the wet fabric.
- Spread the shirt properly-
- Instead of folding, spread the shirt completely to maximize its surface area, allowing more water to evaporate quickly.
- Expose to sunlight-
- Dry the shirt in direct sunlight, as higher temperature accelerates evaporation.
- Use an iron-
- Ironing the wet shirt on a low heat setting evaporates moisture quickly.
- Use a dryer (if available)-
- A tumble dryer with warm air will quickly remove moisture from the fabric.
Conclusion-
By following these steps, the shirt can dry much faster and be ready for the party.
26. Comment on the following statements-
(a) Evaporation produces cooling.
- Evaporation absorbs heat from surroundings, which causes a cooling effect.
- Example- Sweating in hot weather helps cool the body as the sweat evaporates, taking heat from the skin.
(b) Rate of evaporation of an aqueous solution decreases with increase in humidity.
- High humidity means the air already contains a large amount of water vapour, making it harder for water to evaporate.
- Example- Clothes dry faster in dry weather than in humid conditions.
(c) Sponge though compressible, is a solid.
- A sponge has a fixed shape and definite volume, which are properties of solids.
- It can be compressed due to the air trapped in its tiny pores, but it does not change its state, confirming that it is a solid.
27. Why does the temperature of a substance remain constant during its melting point or boiling point?
Answer-
- When a substance reaches its melting or boiling point, it undergoes a phase change.
- At this stage, all the heat energy supplied is used to break intermolecular bonds, rather than increasing the temperature.
- This energy is called latent heat–
- Latent heat of fusion– Energy required to convert a solid into a liquid at its melting point.
- Latent heat of vaporization– Energy required to convert a liquid into gas at its boiling point.
Example-
- Melting of ice at 0°C– Ice continues absorbing heat without temperature change until all the ice turns into water.
- Boiling of water at 100°C– The water temperature remains at 100°C until all the water converts into steam.
Conclusion-
The temperature remains constant during phase changes because energy is used to overcome intermolecular forces rather than increasing kinetic energy.
Also Check – Matter In Our Surroundings – Worksheet with Answer Key


HELLO FOEY! I am Mithi… I FOUND UR WEBSITE… LOVE U!