The Reacting Zinc Granules with Dilute Hydrochloric or Sulfuric Acid activity in the chemistry curriculum of Class 10 is an engaging experiment that demonstrates a single displacement reaction, where zinc metal displaces hydrogen from the acid to form zinc chloride or zinc sulphate and hydrogen gas. This reaction showcases the reactivity of metals and their ability to replace less reactive elements in compounds.
Activity 1.3- Reacting Zinc Granules with Dilute Hydrochloric or Sulfuric Acid
Materials Needed-
- Zinc granules- Serve as the reactive metal in the experiment.
- Dilute hydrochloric or sulfuric acid- The acid reacts with zinc to produce hydrogen gas.
- Safety equipment- Protective goggles, lab coat, and gloves to handle acids safely.
- Test tube or conical flask- To contain the reaction mixture.
Steps of the Activity-
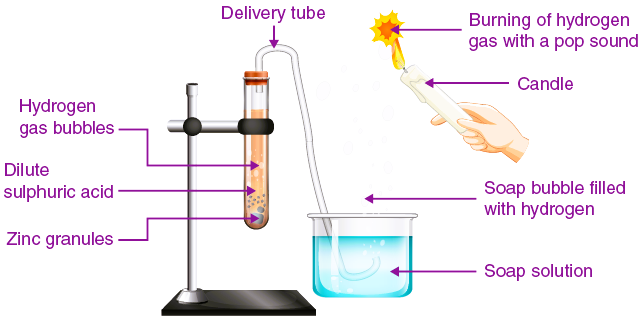
- Preparation- Add a small amount of zinc granules to the test tube or flask.
- Reaction Initiation- Carefully add the dilute acid to the zinc and observe.
- Observation- Look for effervescence indicating the release of hydrogen gas.
What to Look For-
- Bubbles of hydrogen gas forming on the surface of zinc granules.
- The possible increase in temperature, indicating an exothermic reaction.
The reaction between zinc granules and dilute hydrochloric acid is represented by the chemical equation-
Zn (s) + 2HCl (aq) -> ZnCl₂ (aq) + H₂ (g)
For the reaction with dilute sulfuric acid, the equation is-
Zn (s) + H₂SO₄ (aq) -> ZnSO₄ (aq) + H₂ (g)
These reactions illustrate the displacement of hydrogen by zinc,
forming zinc chloride or zinc sulphate and releasing hydrogen gas
Why It Happens-
- Zinc, being more reactive than hydrogen, displaces hydrogen ions from the acid, forming zinc chloride or sulphate and releasing hydrogen gas in the process.
Importance of the Activity-
- Illustrates the concept of single displacement reactions in chemistry.
- Demonstrates the reactivity series of metals, where more reactive metals displace less reactive ones from their compounds.
- Engages with the concept of exothermic reactions, where heat is released as a product of the reaction.
This experiment provides a comprehensive understanding of chemical reactivity, displacement reactions, and the practical application of theoretical concepts in a controlled laboratory setting.