The “Heating Ferrous Sulphate Crystals” activity in the chemistry curriculum of Class 10 is a classic example of a decomposition reaction in the chemistry curriculum. This experiment involves heating ferrous sulphate crystals, which initially are green in colour, and observing the changes that occur as a result of heating.
Activity 1.5 – ‘Heating Ferrous Sulphate Crystals- Decomposition Reaction
Materials Needed-
- Ferrous sulphate crystals- Green-coloured crystals that serve as the reactant.
- Safety equipment– Protective goggles, lab coat, and gloves to ensure safety from the fumes and heat produced.
- Heat source- A Bunsen burner to supply the necessary heat for the reaction.
- A test tube or crucible- To contain the ferrous sulphate crystals during heating.
Steps of the Activity-
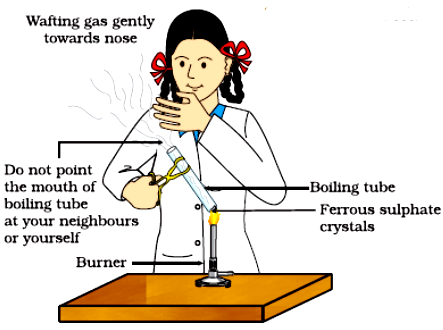
- Preparation- Place a small amount of ferrous sulphate crystals in the test tube or crucible.
- Heating- Gently heat the ferrous sulphate crystals, observing the changes carefully.
- Observation- Note the colour change from green to brown and the evolution of gases.
What to Look For-
- The colour change of the crystals from green to white and then to brown, indicating the decomposition of ferrous sulphate.
- The release of gases, which may include sulphur dioxide and sulphur trioxide, indicating the breakdown of the compound.
Why It Happens-
- Upon heating, ferrous sulphate decomposes in a multi-step process, initially losing water to form anhydrous ferrous sulphate, which further breaks down to iron(III) oxide (Fe2O3), sulphur dioxide (SO2), and sulphur trioxide (SO3).
Chemical Reaction
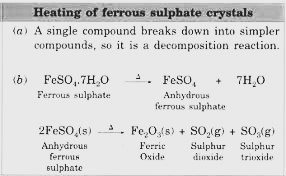
The ‘Heating Ferrous Sulphate Crystals’ activity demonstrates the decomposition reaction-
2FeSO₄ (s) → Heat → Fe₂O₃ (s) + SO₂ (g) + SO₃ (g)
This experiment involves heating green ferrous sulphate crystals, which decompose into brown ferric oxide (Fe₂O₃), sulphur dioxide (SO₂), and sulphur trioxide (SO₃) gases. It showcases thermal decomposition, highlighting the breakdown of a compound due to heat, and emphasises the observable changes such as colour transformation and gas evolution, providing insights into the nature of chemical reactions and the stability of compounds when subjected to thermal energy
This activity vividly demonstrates the concept of decomposition reactions, where a single compound breaks down into two or more simpler substances, often requiring an input of energy in the form of heat. It highlights the thermal decomposition process and the changes that compounds undergo when subjected to high temperatures.