Table of Contents
What Does “Pure Substances” Mean?
Everyday vs Scientific Meaning of Pure Substances
| Everyday Meaning | Scientific Meaning |
| No mixing or adulteration | Made of only one kind of particle |
| Free from harmful substances | Same composition and same properties throughout |
| Examples: Pure milk, pure ghee | Examples: Distilled water, pure iron, oxygen gas |
Explanation:
- In daily life, people call things pure if they look clean or are not mixed with anything else.
Example: If milk is not mixed with water, people call it “pure milk.” - But in science, a pure substance has a fixed composition. It is made up of only one type of particle and has definite properties like boiling point, melting point, etc.
Examples of Scientifically Pure Substances
| Substance | What it contains |
| Distilled water | Only H₂O molecules |
| Iron (Fe) | Only iron atoms |
| Oxygen (O₂) | Only oxygen molecules |
| Carbon dioxide (CO₂) | Only CO₂ molecules |
Substances That Look Pure But Are Not
These are mixtures of different substances even though they may look like one substance.
| Substance | Why it is not pure (scientifically) |
| Milk | Contains water, fat, proteins, minerals – a mixture |
| Mineral water | Has dissolved gases and salts along with water – a mixture |
| Juice | Contains sugar, water, flavour compounds, pulp – a mixture |
| Ghee | May have different fats and sometimes added flavour – not a pure compound |
Key Idea:
In science, pure does not mean clean or healthy.
It means the substance has only one type of particle throughout.
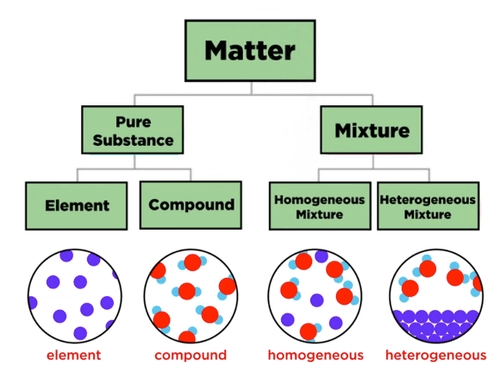
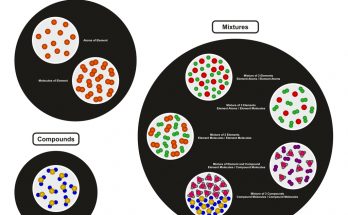
Mixtures
What is a Mixture?
- A mixture is formed when two or more substances are combined physically.
- In mixtures:
- No new substance is formed.
- The substances retain their original properties.
- The proportion of the substances can vary.
Examples:
- Air = Nitrogen + Oxygen + Carbon dioxide (all gases)
- Soil = Sand + Clay + Humus + Stones
- Salt solution = Salt + Water
Characteristics of Mixtures:
- Mixtures do not have fixed composition.
- They can be separated using physical methods like:
- Evaporation
- Filtration
- Magnetic separation
Types of Mixtures
There are two main types of mixtures:
1. Homogeneous Mixtures
- Have a uniform composition throughout.
- You cannot see the different components.
- The substances are evenly mixed at the particle level.
- Also called solutions.
Examples:
- Salt dissolved in water
- Sugar solution
- Air (a mixture of gases)
Characteristics:
- Looks like a single substance
- No visible separation between components
- Even though it looks the same, the concentration can vary
(e.g., one solution may have more salt than another)
2. Heterogeneous Mixtures
- Have a non-uniform composition
- The components do not mix completely
- You can often see the separate substances
- The mixture may look different in different parts
Examples:
- Oil and water
- Soil
- Sand and iron filings
Characteristics:
- Different parts look different
- Particles can be seen (in many cases)
- Can be separated more easily
Comparison Table: Homogeneous vs Heterogeneous Mixtures
| Feature | Homogeneous Mixture | Heterogeneous Mixture |
| Composition | Uniform throughout | Non-uniform |
| Appearance | Looks like one substance | Looks like a mix of substances |
| Visibility of particles | Not visible | Often visible |
| Examples | Salt in water, air, sugar solution | Oil and water, sand and iron, soil |
| Separation of components | Difficult | Often easy (e.g., using filters/magnets) |
| Also called | Solutions | Not called solutions |
Differentiate between Homogeneous and Heterogeneous mixture with examples.- In details
What is a Solution?
Definition
A solution is a homogeneous mixture of two or more substances. The substances are evenly mixed at the particle level and cannot be seen separately. A solution appears to be a single substance.
Examples:
Salt water, lemonade, soda water, air.
Components of a Solution
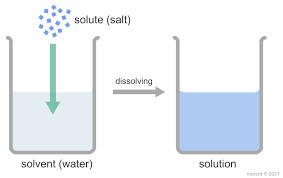
- Solvent – The substance that dissolves the other. It is usually present in a larger amount.
- Solute – The substance that gets dissolved in the solvent. It is present in a smaller amount.
Example:
In a salt solution, water is the solvent and salt is the solute.
Also Check – Solute, Solvent and Solution- Definition, Difference between, Characteristics, Examples
Types of Solutions (based on physical states)
- Solid in liquid – Sugar in water, salt in water
- Gas in liquid – Carbon dioxide in soda water
- Gas in gas – Air (mixture of gases like oxygen, nitrogen, etc.)
- Solid in solid – Alloys like brass (zinc mixed with copper)
Properties of a Solution
- It is homogeneous (uniform throughout)
- Solute particles are too small to be seen
- Does not scatter light (no Tyndall effect)
- Solute cannot be separated by filtration
- Stable – solute does not settle down over time
Concentration of a Solution
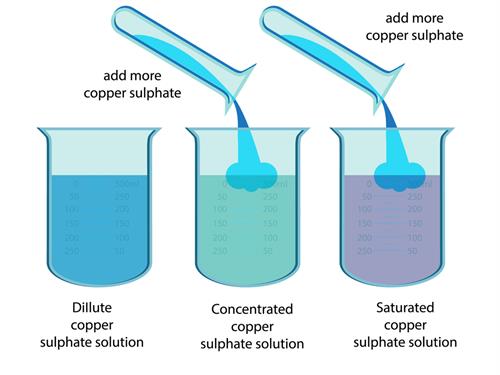
Types of Concentration (Qualitative)
- Dilute solution – Small amount of solute
- Concentrated solution – Large amount of solute
- Saturated solution – No more solute can dissolve at a given temperature
- Unsaturated solution – More solute can still dissolve
Solubility
Solubility is the maximum amount of solute that can dissolve in a fixed amount of solvent at a given temperature.
- Different substances have different solubilities
- Solubility generally increases with temperature
Methods of Expressing Concentration (Quantitative)
1. Mass by Mass Percentage (%)
= (Mass of solute / Mass of solution) × 100
2. Mass by Volume Percentage (%)
= (Mass of solute / Volume of solution) × 100
3. Volume by Volume Percentage (%)
= (Volume of solute / Volume of solution) × 100
Example Calculation
A solution contains 40 g of salt in 320 g of water.
Find the mass by mass percentage.
- Mass of solute = 40 g
- Mass of solvent = 320 g
- Mass of solution = 40 + 320 = 360 g
Mass % = (40 / 360) × 100 = 11.1%
What is a Suspension?
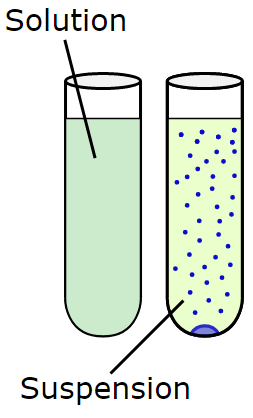
Definition
A suspension is a heterogeneous mixture in which solid particles are spread out (dispersed) in a liquid, but they do not dissolve in the liquid.
- The particles in a suspension are large enough to be seen with the naked eye.
- The particles are not uniformly distributed, and they may settle down if the suspension is left undisturbed.
- A suspension can be filtered to separate the solid particles.
Examples of Suspensions
| Example | Solid (solute) | Liquid (solvent) |
| Muddy water | Soil or clay | Water |
| Sand in water | Sand | Water |
| Chalk powder in water | Chalk particles | Water |
| Flour in water | Wheat flour | Water |
Properties of Suspensions
- It is a heterogeneous mixture.
- The particles are visible to the naked eye.
- Particles are large enough to settle at the bottom when left undisturbed.
- It is unstable — the components separate on standing.
- The mixture can be separated by filtration.
- It shows the Tyndall effect — a beam of light becomes visible when passed through the mixture.
Understanding with an Example
Imagine you mix sand in a glass of water. At first, it may seem like a mixture, but after a few minutes, the sand settles at the bottom. This is a suspension. You can easily separate the sand by pouring out the water or by using filter paper.
Comparison Table: Solution vs Suspension
| Feature | Solution | Suspension |
| Type of mixture | Homogeneous | Heterogeneous |
| Particle visibility | Not visible | Visible to the naked eye |
| Particle size | Very small (< 1 nm) | Large (> 1000 nm) |
| Settling of particles | Do not settle | Settle down when left undisturbed |
| Filtration | Cannot be filtered | Can be filtered |
| Tyndall effect | Does not show | Shows (beam of light becomes visible) |
| Stability | Stable | Unstable |
What is a Colloid
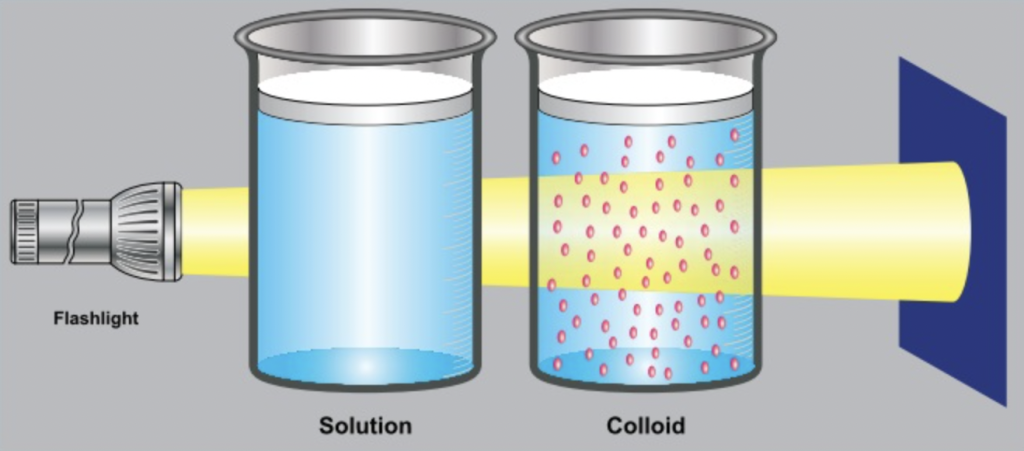
Definition
A colloid is a type of heterogeneous mixture that appears to be homogeneous because the particles are very small and evenly spread out. The particles don’t settle and cannot be seen by naked eyes.
Although it looks uniform like a solution, it is actually heterogeneous at the particle level.
Key Characteristics
- Particles are small but not as small as in a solution.
- They do not settle down on standing.
- Cannot be filtered using normal filter paper.
- They scatter light (Tyndall effect), making the light beam visible.
Components of a Colloid
- Dispersed phase – The solute-like component (the particles)
- Dispersion medium – The solvent-like component (the medium)
Example: In milk, fat droplets are the dispersed phase, and water is the dispersion medium.
Tyndall Effect in Colloids
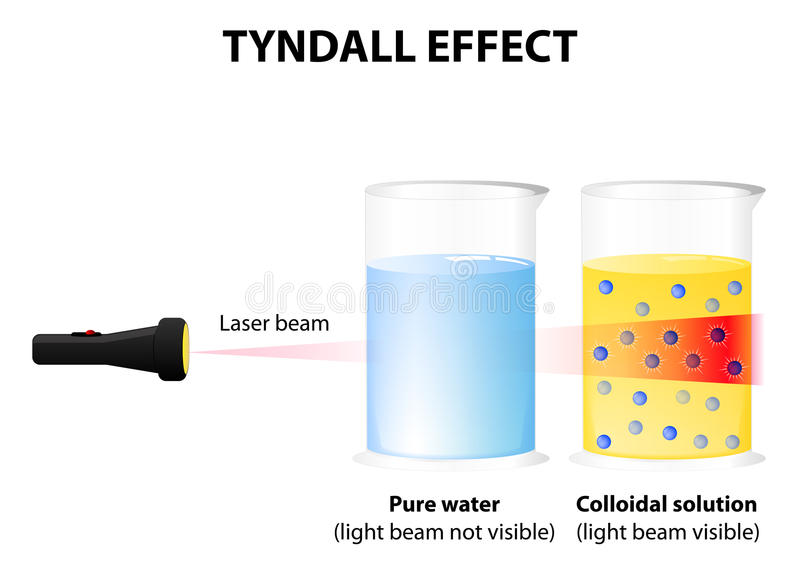
The Tyndall effect is the scattering of light by colloidal particles.
You can see this when sunlight passes through mist or a beam of torchlight passes through smoke or fog.
Examples of Colloids
| Type of Colloid | Dispersed Phase | Dispersion Medium | Example |
| Aerosol | Liquid or solid | Gas | Fog, clouds, smoke |
| Foam | Gas | Liquid or solid | Shaving cream, sponge |
| Emulsion | Liquid | Liquid | Milk, face cream |
| Sol | Solid | Liquid | Mud, paint, milk of magnesia |
| Gel | Liquid | Solid | Jelly, cheese, butter |
| Solid Sol | Solid | Solid | Colored glass, alloys |
Properties of Colloids
- Heterogeneous mixture (but appears homogeneous)
- Particles are not visible to the naked eye
- Stable (particles do not settle)
- Cannot be filtered by ordinary filter paper
- Show Tyndall effect (scatter light)
- Separated using centrifugation, not filtration
Comparison: Solution vs Suspension vs Colloid
| Feature | Solution | Suspension | Colloid |
| Type of mixture | Homogeneous | Heterogeneous | Heterogeneous |
| Particle size | Very small (< 1 nm) | Large (> 1000 nm) | Intermediate (1–1000 nm) |
| Particle visibility | Not visible | Visible | Not visible |
| Settling of particles | No | Yes | No |
| Filtration | Cannot be filtered | Can be filtered | Cannot be filtered |
| Tyndall effect | No | Yes | Yes |
| Stability | Stable | Unstable | Quite stable |
Separation of Mixtures
Mixtures are made up of two or more substances that are physically combined and can be separated by various physical methods. The method used depends on the type of mixture and the properties of its components.
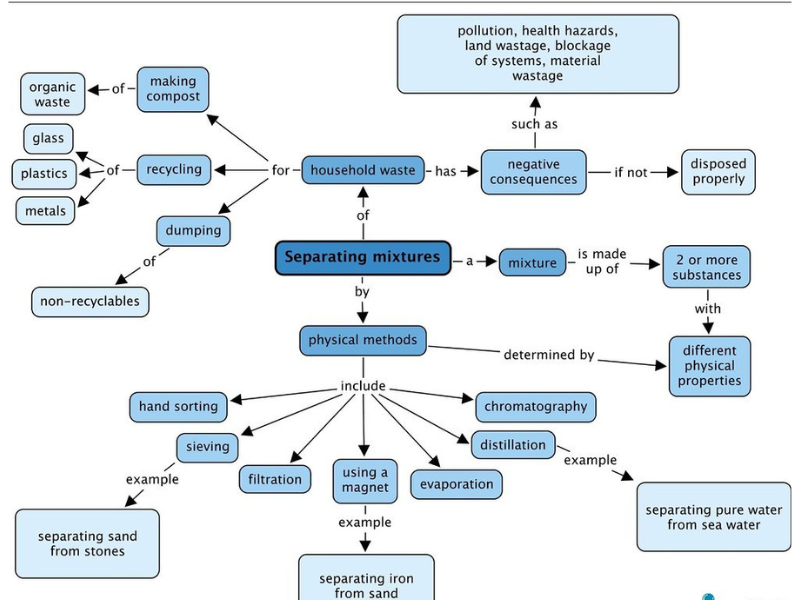
Methods of Separation of Mixtures
1. Evaporation
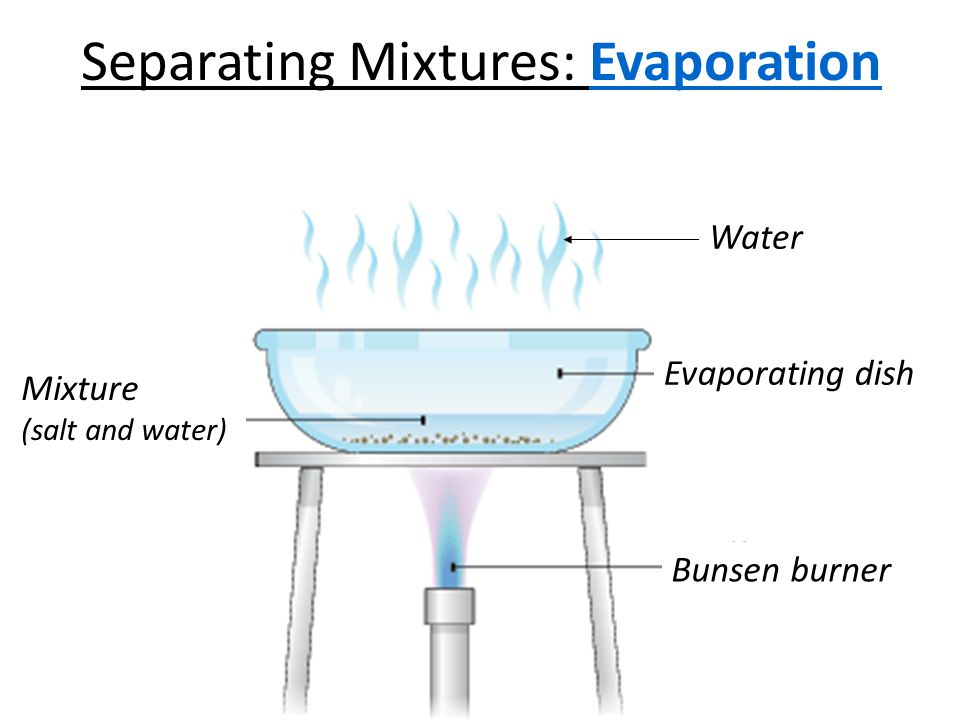
- Definition: A method used to separate a dissolved solid from a liquid by heating the mixture until the liquid evaporates.
- Principle: The liquid changes into vapor, leaving the solid behind.
- Example: Separating salt from saltwater by heating the water until it evaporates, leaving salt behind.
2. Filtration
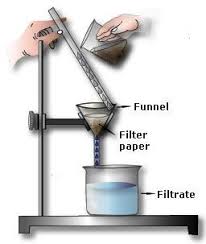
- Definition: A process used to separate an insoluble solid from a liquid using a filter.
- Principle: The liquid passes through the filter paper, while the solid particles remain on the filter.
- Example: Separating tea leaves from tea using a strainer.
3. Centrifugation
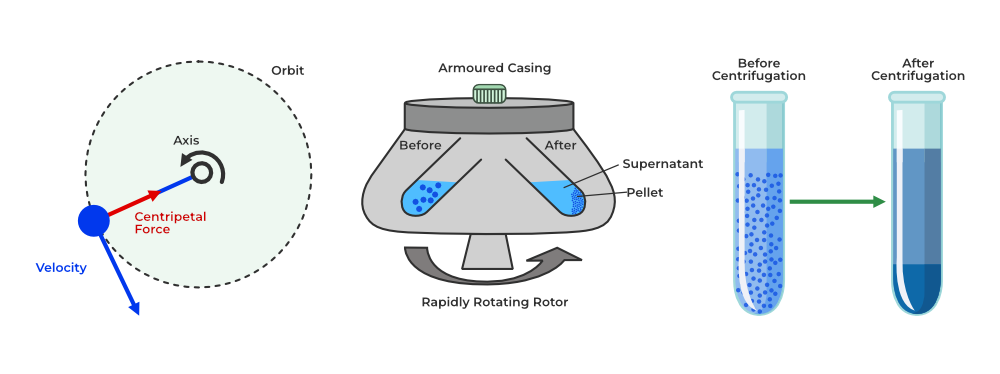
- Definition: A method used to separate particles of different densities in a liquid using rapid spinning.
- Principle: The denser particles move outward due to the spinning force, and the lighter particles stay at the center.
- Example: Separating cream from milk by spinning the milk at high speed.
4. Chromatography
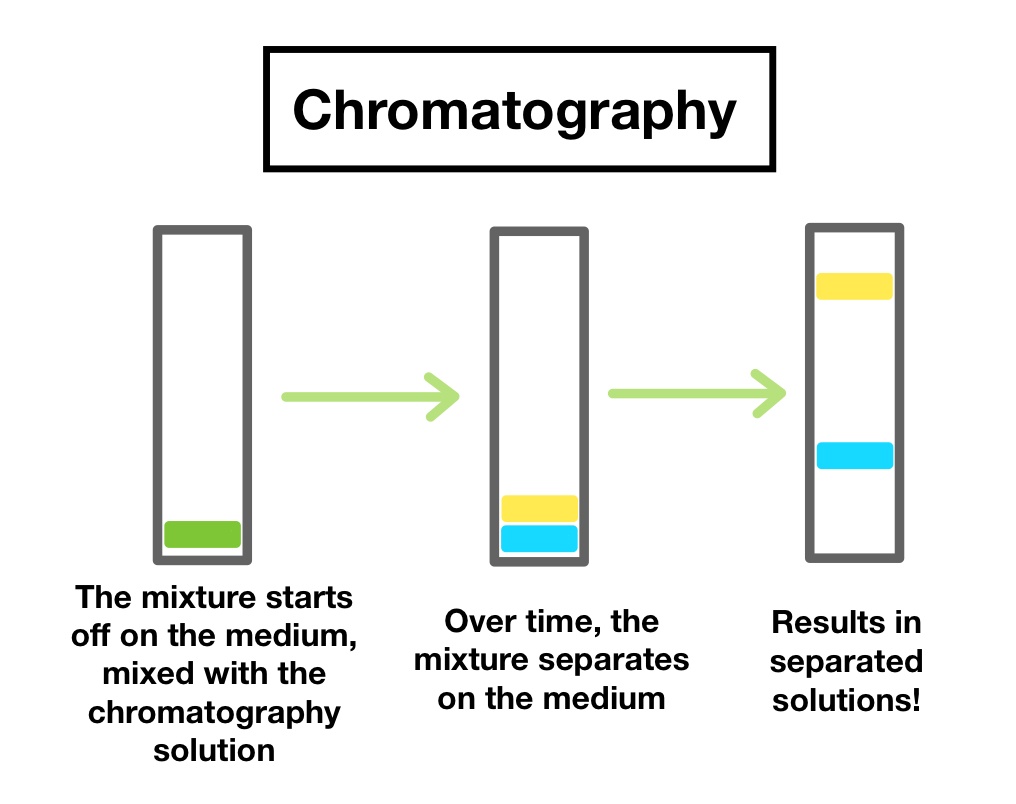
- Definition: A technique used to separate the different components of a liquid mixture based on how they move through a medium.
- Principle: Different substances travel at different speeds through a stationary phase (paper or gel).
- Example: Separating different pigments in ink by placing a drop of ink on filter paper and dipping it in water.
5. Distillation
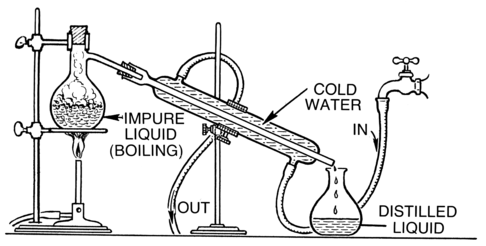
- Definition: A method used to separate two liquids with different boiling points by heating and condensing the vapor.
- Principle: The component with the lower boiling point evaporates first and is then condensed back into liquid form.
- Example: Separating alcohol from water in the alcohol industry.
6. Sublimation
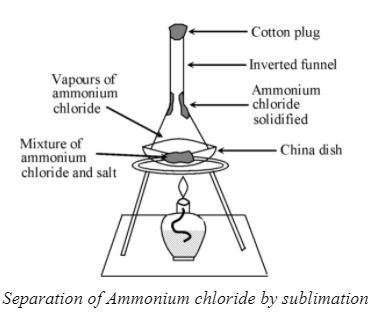
- Definition: A process where a solid changes directly into gas without passing through the liquid state.
- Principle: Only substances that can sublimate can be separated using this method.
- Example: Separating ammonium chloride from salt because ammonium chloride turns into gas upon heating while salt remains as a solid.
7. Magnetic Separation
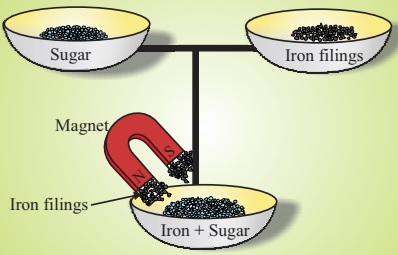
- Definition: A method used to separate magnetic substances from non-magnetic ones.
- Principle: A magnet attracts magnetic materials and leaves behind the non-magnetic ones.
- Example: Separating iron filings from a mixture of sand and iron using a magnet.
8. Decantation and Sedimentation

- Definition: A method used to separate an insoluble solid from a liquid by letting the heavier particles settle at the bottom.
- Principle: Heavier particles settle due to gravity (sedimentation), and the clear liquid is poured off (decantation).
- Example: Separating sand from water by allowing the sand to settle at the bottom before pouring out the clear water.
Summary of Separation Methods
| Method | Used to Separate | Example |
| Evaporation | Solute from solvent (solution) | Salt from seawater |
| Filtration | Insoluble solid from liquid | Tea leaves from tea |
| Centrifugation | Different densities in a liquid | Cream from milk |
| Chromatography | Different colors/pigments in a liquid | Colors in ink |
| Distillation | Two liquids with different boiling points | Alcohol from water |
| Sublimation | A sublimable solid from a non-sublimable solid | Ammonium chloride from salt |
| Magnetic Separation | Magnetic from non-magnetic substances | Iron filings from sand |
| Decantation & Sedimentation | Heavy solids from a liquid | Sand from water |
Changes in Matter
2.3 Physical and Chemical Changes
What is a Physical Change?
A physical change is a change in which:
- The form or appearance of a substance may change,
- But its chemical composition remains the same.
There is no new substance formed.
Key Features of Physical Change:
- Reversible in many cases
- No new substance is formed
- Only physical properties change, such as:
- Shape
- Size
- State (solid, liquid, gas)
- Texture
- Volume
- Colour (sometimes)
Examples of Physical Changes:
- Melting of butter
- Boiling of water into steam
- Freezing of water into ice
- Breaking glass
- Dissolving salt in water
- Cutting of wood or paper
Even though dissolving seems like a change, it does not create a new substance — the solute can be recovered by physical methods (like evaporation).
Also Check – 5 Fun Facts About The Physical Properties Of Matter
What is a Chemical Change?
A chemical change is a change in which:
- The chemical composition of the substance changes
- A new substance with different properties is formed
These changes are often irreversible by simple physical means.
Key Features of Chemical Change:
- New substance is formed
- Often irreversible
- Energy is absorbed or released (heat, light, sound)
- Properties of the new substance are different
- Sometimes you may observe:
- Change in colour
- Formation of gas
- Formation of a precipitate
- Change in temperature
Examples of Chemical Changes:
- Burning of wood or paper
- Rusting of iron
- Cooking of food
- Digestion of food
- Souring of milk
- Mixing vinegar and baking soda (releases CO₂ gas)
Also Check – Properties of Matter: Physical, Chemical, and Functional Explained With Examples
Comparison: Physical Change vs Chemical Change
| Feature | Physical Change | Chemical Change |
| Composition | Does not change | Changes |
| New substance formed | No | Yes |
| Reversibility | Usually reversible | Usually irreversible |
| Examples | Melting, boiling, cutting, dissolving | Burning, rusting, cooking, rotting |
| Properties changed | Only physical properties | Both physical and chemical properties |
| Energy change | Usually low or none | Often involves energy release or absorption |
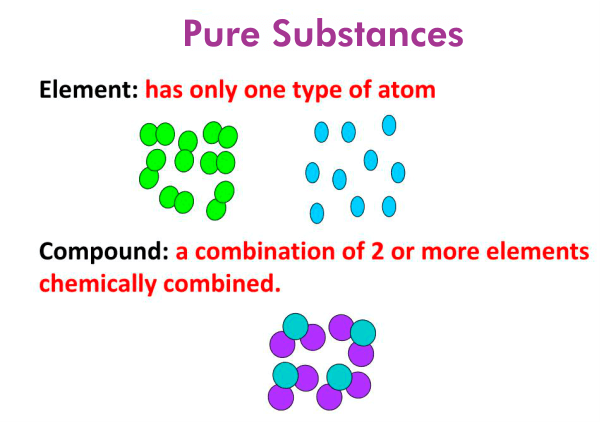
Pure Substances
Types of Pure Substances
A pure substance is made up of only one kind of particle and has a fixed composition. It cannot be separated into other substances by physical methods.
Pure substances are classified into two main types:
Elements
Compounds (covered in the next section)
Elements
Definition
An element is a basic form of matter that cannot be broken down into simpler substances by any chemical reaction.
- Each element is made up of only one kind of atom.
- It has a definite set of properties.
- Currently, more than 100 elements are known, out of which 92 are naturally occurring.
Also Check – What Are Elements, Compounds, and Mixtures? Key Differences and Examples
Also Check- The Periodic Table of Elements: What It Is and How It Works
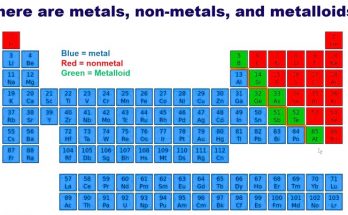
Categories of Elements
Elements are grouped into three main categories based on their properties:
1. Metals

Metals are usually hard, shiny, and good conductors of heat and electricity.
Examples:
Gold, copper, iron, silver, aluminum
Properties of Metals:
- Have lustre (shiny appearance)
- Good conductors of heat and electricity
- Ductile (can be drawn into wires)
- Malleable (can be hammered into thin sheets)
- Sonorous (make ringing sound when hit)
- Mostly solid at room temperature (except mercury)
2. Non-Metals

Non-metals are elements that do not have the properties of metals.
Examples:
Oxygen, bromine, chlorine, carbon
Properties of Non-Metals:
- Usually not shiny
- Poor conductors of heat and electricity
- Brittle if solid (not ductile or malleable)
- Many are gases or liquids at room temperature
- Do not produce sound when hit (non-sonorous)
3. Metalloids
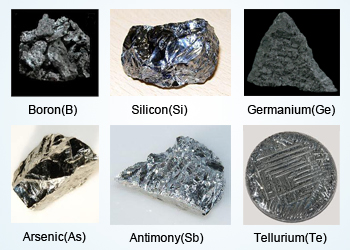
Metalloids have properties of both metals and non-metals.
They are also called semi-metals.
Examples:
Silicon, boron, germanium
Properties of Metalloids:
- Semiconductors of electricity
- Have intermediate properties
- Used widely in electronics
Comparison Table: Metals vs Non-Metals
| Property | Metals | Non-Metals |
| Appearance | Shiny (lustrous) | Dull |
| Conductivity | Good conductors | Poor conductors |
| Malleability | Can be hammered into sheets | Brittle, cannot be shaped easily |
| Ductility | Can be drawn into wires | Not ductile |
| State at room temperature | Mostly solid (except mercury) | Solid, liquid or gas |
| Sound | Sonorous (make ringing sound) | Non-sonorous |
| Examples | Iron, copper, gold, aluminum | Oxygen, bromine, sulfur, chlorine |
Compounds
Definition
A compound is a pure substance that is formed when two or more elements are chemically combined in a fixed proportion by mass.
- The elements in a compound lose their individual properties.
- A compound has new and uniform properties.
- The components cannot be separated by physical methods.
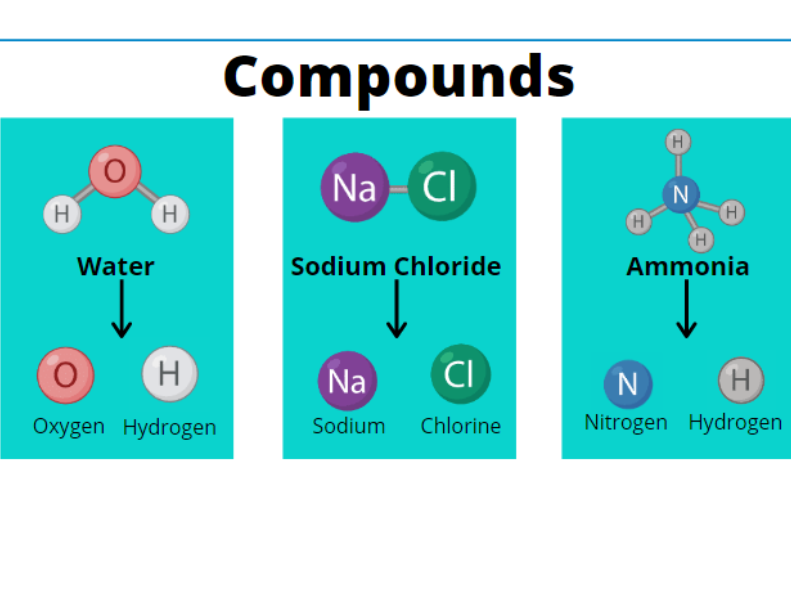
Key Characteristics of Compounds
- Made of two or more elements chemically bonded together.
- Have a fixed composition by mass.
- Always form a new substance with different properties from the original elements.
- Can be broken down only by chemical reactions (not by filtration, evaporation, etc.).
- Show uniform properties throughout.
Examples of Compounds
| Compound | Elements Combined | Properties |
| Water (H₂O) | Hydrogen + Oxygen | Colourless liquid; puts out fire |
| Carbon dioxide (CO₂) | Carbon + Oxygen | Colourless gas; used in soft drinks |
| Sodium chloride (NaCl) | Sodium + Chlorine | White salt; used in food |
| Iron sulfide (FeS) | Iron + Sulfur | Black solid; non-magnetic |
In each of these, the new compound has completely different properties than the elements used to form it.
How Compounds are Different from Mixtures
| Property | Compound | Mixture |
| Formation | Elements react chemically | Substances are mixed physically |
| Composition | Fixed proportion | Variable proportion |
| Properties | New properties different from individual elements | Shows properties of each component |
| Separation methods | Cannot be separated physically | Can be separated by physical methods |
| Energy change | Energy is often involved in formation | Usually no energy change during mixing |
| Uniformity | Homogeneous | Can be homogeneous or heterogeneous |