Long Answer Questions with Answers (Extra Questions)
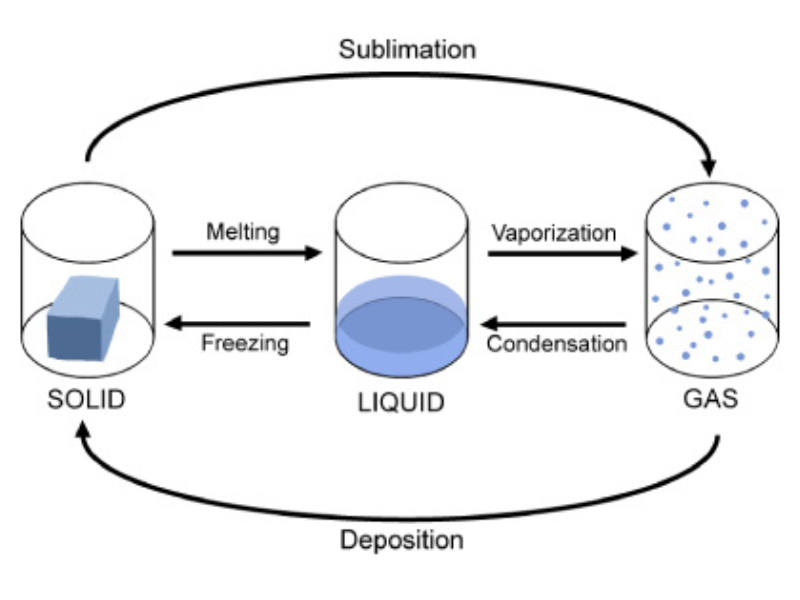
1. Explain in detail the interconversion of states of matter. How can we change one state of matter into another? Support your answer with a diagram.
Answer:
Interconversion of states of matter refers to the process where one state of matter (solid, liquid, or gas) changes into another due to changes in temperature or pressure.
Processes of Interconversion:
- Melting (Fusion): Solid → Liquid (Occurs when heat is supplied, weakening intermolecular forces).
- Freezing (Solidification): Liquid → Solid (Occurs when heat is removed, strengthening intermolecular forces).
- Boiling (Vaporization): Liquid → Gas (Occurs when heat is supplied until the boiling point is reached).
- Condensation: Gas → Liquid (Occurs when heat is removed, allowing gas particles to come closer).
- Sublimation: Solid → Gas (Some substances like dry ice, camphor, and naphthalene bypass the liquid state).
- Deposition: Gas → Solid (Reverse of sublimation, e.g., frost formation).
✅ Effect of Temperature: Increasing temperature increases kinetic energy, reducing intermolecular forces and causing phase transitions.
✅ Effect of Pressure: Increasing pressure can compress gases into liquids and solids (e.g., liquefied petroleum gas – LPG).
Diagram:
2. Explain why evaporation causes cooling. What are the factors that affect the rate of evaporation?
Answer:
Evaporation is the process by which liquid particles at the surface gain enough energy to escape into the gas phase below their boiling point.
✔️ Why Evaporation Causes Cooling?
- The molecules with the highest kinetic energy escape first, reducing the average kinetic energy of the remaining molecules.
- As a result, the temperature of the liquid decreases, causing a cooling effect.
- This is why sweating cools our body, and why water sprinkled on the floor cools a room in summer.
Factors Affecting Evaporation:
- Surface Area: Larger surface area increases evaporation (e.g., clothes dry faster when spread out).
- Temperature: Higher temperature provides more kinetic energy, increasing evaporation.
- Humidity: Higher humidity slows evaporation as the air is already saturated with water vapor.
- Wind Speed: Faster wind removes vapor molecules, increasing evaporation rate.
- Nature of Liquid: Volatile liquids (e.g., acetone) evaporate faster than less volatile liquids (e.g., water).
Also Check – Chapter 1 -MATTER IN OUR SURROUNDINGS -Class 9 – Simplified Notes
3. Differentiate between boiling and evaporation in terms of process, energy requirements, and occurrence.
Answer:
| Feature | Boiling | Evaporation |
| Definition | Rapid conversion of liquid to gas at boiling point. | Slow surface-level conversion of liquid to gas below boiling point. |
| Temperature | Occurs at a fixed temperature (boiling point). | Occurs at any temperature. |
| Location | Takes place throughout the liquid. | Happens only at the surface. |
| Energy Source | Requires continuous heat supply. | Uses existing heat from surroundings. |
| Speed | Rapid process. | Slow process. |
| Example | Boiling water in a kettle. | Water drying from wet clothes. |
✅ Key Difference: Boiling is a bulk phenomenon (entire liquid changes to gas), while evaporation is a surface phenomenon (only surface molecules escape).
Also Check – Matter In Our Surroundings – Worksheet with Answer Key
4. Explain why gases are more compressible than solids and liquids. Use kinetic theory to support your answer.
Answer:
✔️ According to Kinetic Theory of Matter:
- Matter consists of tiny particles that are always in motion.
- The amount of space between particles determines the compressibility of a substance.
✔️ Why Gases Are Highly Compressible:
- Large Intermolecular Space:
- Gas particles are widely spaced, allowing them to be compressed easily.
- Weak Intermolecular Forces:
- Unlike solids and liquids, gases have negligible attractive forces.
- Random Motion of Particles:
- Gas molecules move freely, which allows them to be compressed into smaller volumes.
- Practical Applications:
- LPG cylinders store gas in compressed form for easy transportation.
- Oxygen cylinders in hospitals contain compressed oxygen for medical use.
✅ Solids and Liquids:
- Solids are almost incompressible due to tightly packed particles.
- Liquids are slightly compressible but not as much as gases.
5. Why does a gas exert pressure on the walls of its container? Explain using kinetic molecular theory.
Answer:
✔️ According to Kinetic Molecular Theory:
- Gas particles move randomly in all directions at high speed.
- When they collide with the walls of a container, they exert force on it.
- The cumulative effect of these collisions results in gas pressure.
Key Factors Affecting Gas Pressure:
- Number of Particles: More gas particles increase the number of collisions, increasing pressure.
- Temperature: Higher temperature increases kinetic energy, making particles collide more forcefully, increasing pressure.
- Volume: Decreasing the container’s volume forces particles closer, increasing pressure (Boyle’s Law).
✅ Example:
- A fully inflated balloon is firm because air inside exerts pressure on the balloon’s walls.
6. Why do we see water droplets on the outer surface of a glass containing ice-cold water? What is this process called?
Answer:
✔️ The phenomenon is called condensation.
✔️ Reason:
- Air contains water vapor in the form of moisture.
- When humid air comes in contact with the cold surface of the glass, the air loses heat.
- The water vapor in the air loses kinetic energy and condenses into tiny liquid droplets.
✅ Everyday Examples of Condensation:
- Fogging on a bathroom mirror after a hot shower.
- Dew forming on leaves in the early morning.
Also Check – 82 Reasoning-Based Questions & Answers-Matter In Our Surroundings -Class 9 Science
7. Why is ice at 0°C more effective in cooling than water at 0°C?
Answer:
✔️ Ice at 0°C absorbs more heat than water at 0°C because of latent heat of fusion.
✔️ Explanation:
- When ice melts, it absorbs latent heat of fusion (334 J/g) from the surroundings.
- This extra heat absorption cools the surrounding environment.
- Water at 0°C, however, is already in a liquid state and does not require additional heat absorption.
✅ Example:
- Ice packs are used to reduce swelling because ice absorbs more heat and cools faster.
Also Check – Rapid Revision –Chapter 1- Matter In Our Surroundings – Class 9 Science
8. Why does steam cause more severe burns than boiling water at 100°C?
Answer:
✔️ Steam has more energy than boiling water due to latent heat of vaporization.
✔️ Explanation:
- Both steam and boiling water are at 100°C, but steam contains extra energy in the form of latent heat of vaporization (2260 J/g).
- When steam condenses back to water on the skin, it releases this extra heat, causing severe burns.
✅ Example:
- Steam burns from a pressure cooker are more painful than spilling boiling water on the skin.

