What Are Elements, Compounds, and Mixtures? Key Differences and Examples
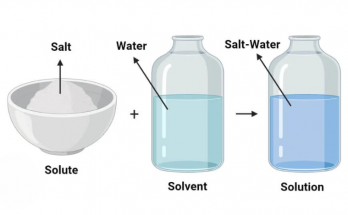
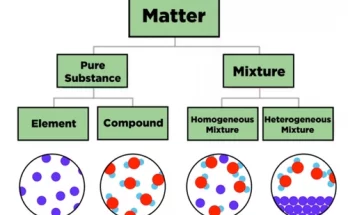
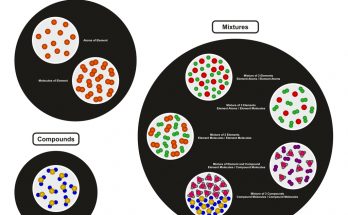
Matter is the substance that forms everything around us. It can be classified into elements, compounds, and mixtures. Elements contain only one type of atom, compounds are chemically bonded atoms in fixed ratios and mixtures are physical combinations of substances. These forms are fundamental in chemistry, as it reveals how substances interact.
What Are Elements, Compounds, and Mixtures? Key Differences and Examples Read More