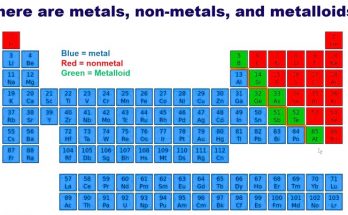
What Are Chemical Elements? A Complete Guide for Students
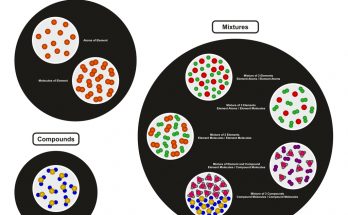
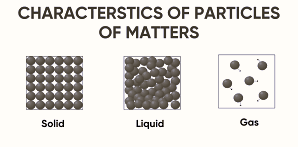
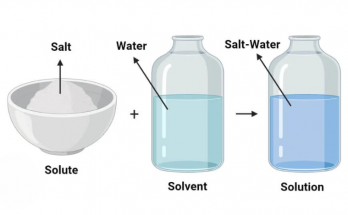
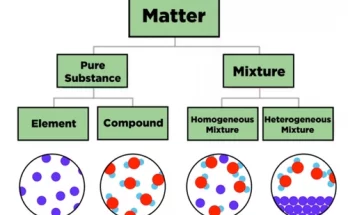
Chemical elements are the basic building blocks of all matter. This guide explains atoms, elements, and the periodic table in simple terms for students. Learn how elements combine to form compounds, how they appear in nature, and how stars create them. Ideal for high school learners exploring chemistry fundamentals and real-life applications of science.
What Are Chemical Elements? A Complete Guide for Students Read More