Table of Contents
Latent Heat
Latent heat is the energy required to change the phase of a substance without changing its temperature. It is categorized into:
- Latent Heat of Fusion – The heat required to convert a solid into a liquid at a constant temperature.
- Latent Heat of Vaporization – The heat required to convert a liquid into a gas at a constant temperature.
These phase transitions occur because the energy is used to break or form intermolecular bonds rather than increasing temperature.
Latent Heat of Fusion (Melting & Freezing)
What is Latent Heat of Fusion?
The latent heat of fusion (also called enthalpy of fusion) is the amount of heat energy required to convert 1 kg of a solid into a liquid at a constant temperature and pressure.
Example of Latent Heat of Fusion
Imagine you have a block of ice at 0°C. If you provide heat, the ice will start melting, but surprisingly, the temperature remains at 0°C until all of the ice has turned into water.
- The energy you provide does not increase the temperature; instead, it is used to break the strong intermolecular bonds that hold the solid (ice) together.
- Only after all the ice has melted will the temperature of the water start rising above 0°C.
Formula for Latent Heat of Fusion
To calculate the heat required to melt a solid, we use the formula:
Q = m × Lf
Where:
- Q = Heat energy required (in Joules, J)
- m = Mass of the substance (in kg)
- Lf = Latent heat of fusion (in J/kg)
Also Check – Latent Heat of Fusion Formula- Easy Explanation with Formula & Examples
Latent Heat of Fusion of Water
For water, the latent heat of fusion is 333.55 kJ/kg, which means:
- 333,550 Joules of energy are needed to convert 1 kg of ice into water at 0°C.
- Even though heat is continuously supplied, the temperature does not increase until all the ice melts.
Why Does Temperature Stay Constant During Melting?
This happens because the heat energy provided is not used to raise the temperature but is instead used to break the strong bonds between the molecules in the solid.
- In a solid, molecules are tightly packed together.
- In a liquid, molecules move freely but are still connected by weaker forces.
- The heat energy is required to overcome these strong forces and convert the solid into a liquid.
- Once all the bonds are broken, any additional heat starts raising the temperature of the liquid.
This is why adding heat to melting ice does not make the ice-water mixture hotter—it just continues melting the ice.
Heat of Solidification (Freezing) – The Reverse Process
The heat of solidification is the opposite of latent heat of fusion.
- When a liquid cools and freezes into a solid, it releases the same amount of heat that was absorbed during melting.
- This heat is released into the surroundings.
Example of Solidification
- 1 kg of water at 0°C freezes into ice, releasing 333.55 kJ of heat into the surroundings.
- That is why frozen lakes help keep surrounding areas slightly warmer—as water freezes, it releases heat into the atmosphere.
Latent Heat of Vaporization (Boiling & Condensation)
What is Latent Heat of Vaporization?
The latent heat of vaporization is the amount of heat energy required to convert 1 kg of a liquid into a gas at a constant temperature and pressure.
Example of Latent Heat of Vaporization
Imagine you are boiling water at 100°C. Even if you keep applying heat, the temperature does not rise above 100°C until all the water turns into steam.
- The heat energy is not used to increase temperature but to break intermolecular bonds and turn the liquid into a gas.
- Once all the water has evaporated, additional heat will then start raising the temperature of the steam.
Formula for Latent Heat of Vaporization
To calculate the heat required to convert a liquid into a gas, we use the formula:
Q = m × Lv
Where:
- Q = Heat energy required (in Joules, J)
- m = Mass of the substance (in kg)
- Lv = Latent heat of vaporization (in J/kg)
Latent Heat of Vaporization of Water
For water, the latent heat of vaporization is 2260 kJ/kg, meaning:
- 2,260,000 Joules of energy are needed to convert 1 kg of water into steam at 100°C.
- Even though heat is continuously supplied, the temperature does not increase until all the water evaporates.
Why Does Temperature Stay Constant During Boiling?
- In a liquid, molecules are held together by intermolecular forces.
- In a gas, molecules move freely without any significant attraction between them.
- The heat energy is required to overcome these forces and convert the liquid into gas.
- Once all the molecules have separated into gas, any additional heat starts raising the temperature of the steam.
This is why boiling water stays at 100°C even when heat is applied continuously.
Latent Heat of Condensation – The Reverse Process
The latent heat of condensation is the opposite of latent heat of vaporization.
- When a gas cools and condenses into a liquid, it releases the same amount of heat that was absorbed during vaporization.
Example of Condensation
- 1 kg of steam at 100°C condenses into water, releasing 2260 kJ of heat into the surroundings.
- This is why steam burns are more dangerous than boiling water burns—steam carries extra heat energy due to latent heat.
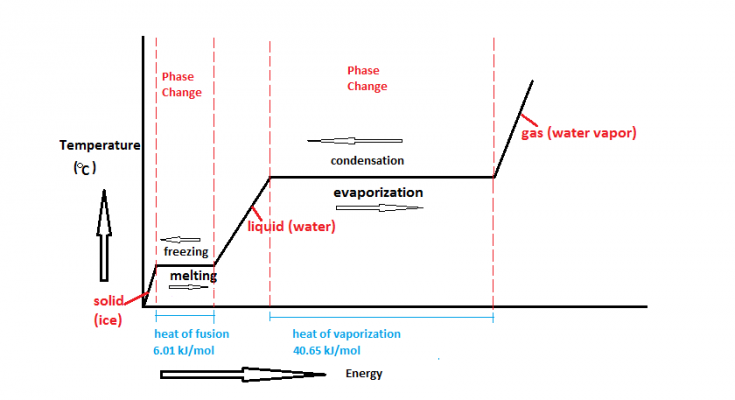
Heating Curve and Phase Changes
The heating curve explains how substances absorb heat during phase changes.
Graph of Latent Heat of Fusion and Vaporization
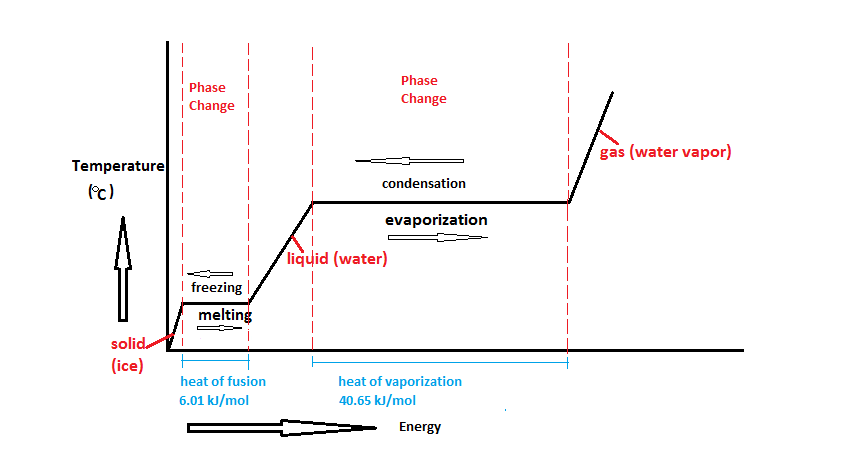
Understanding the Graph
| Stage | Process | Temperature Change? | Heat Energy Used For? |
| 1 | Heating Solid | Yes | Increasing kinetic energy |
| 2 | Melting (Solid → Liquid) | No | Breaking solid bonds (Latent Heat of Fusion) |
| 3 | Heating Liquid | Yes | Increasing kinetic energy |
| 4 | Boiling (Liquid → Gas) | No | Breaking liquid bonds (Latent Heat of Vaporization) |
| 5 | Heating Gas | Yes | Increasing kinetic energy |
- Flat Lines = Latent heat is absorbed, but temperature stays constant.
- Sloped Lines = Sensible heat is absorbed, causing temperature to increase.
Numerical Problems
Problem 1: Latent Heat of Fusion
Question:
How much heat energy is required to melt 2 kg of ice at 0°C?
Given:
- Latent heat of fusion (Lf) = 333.55 kJ/kg
- Mass (m) = 2 kg
Solution:
Q = m * Lf
Q = 2 * 333.55
Q = 667.1 kJ
Answer: 667.1 kJ of heat is required.
Problem 2: Latent Heat of Vaporization
Question:
How much heat energy is required to convert 3 kg of water at 100°C into steam?
Given:
- Latent heat of vaporization (Lv) = 2260 kJ/kg
- Mass (m) = 3 kg
Solution:
Q = m * Lv
Q = 3 * 2260
Q = 6780 kJ
Answer: 6780 kJ of heat is required.
Concept-Based FAQs (Frequently Asked Questions)
1. Why does steam cause more severe burns than boiling water?
Steam carries extra energy due to latent heat of vaporization, which is released upon contact with the skin, causing more severe burns.
Also Check – Why Steam Burns Are More Dangerous Than Boiling Water Burn – In Details
2. Why does ice at 0°C feel colder than water at 0°C?
Ice absorbs latent heat of fusion from your body to melt, while water does not, making ice feel colder.
3. Why does sweating cool the body?
Sweat absorbs latent heat of vaporization from the skin to evaporate, removing heat and cooling the body.
4. Why do phase changes occur at constant temperature?
The heat energy is used to break or form intermolecular bonds, not to increase temperature.
5. Why do refrigerants work based on latent heat?
Refrigerants absorb latent heat of vaporization to cool spaces and release latent heat of condensation to expel heat.
Also Check – Why is the Latent Heat of Steam So High? Find the Answer