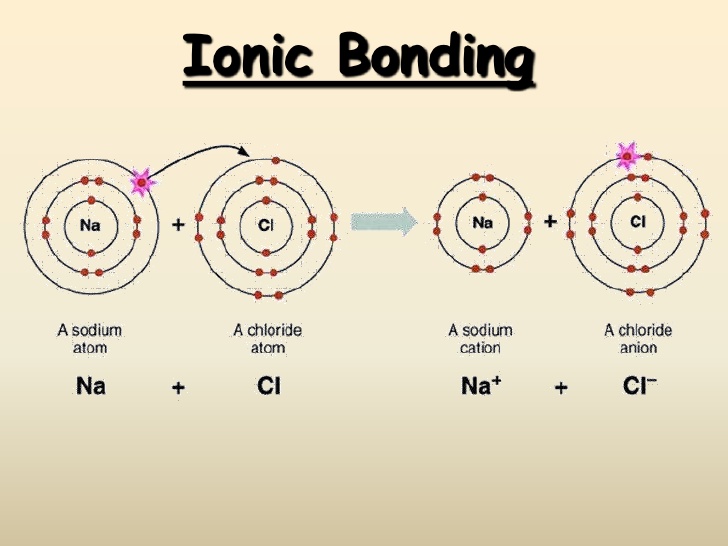
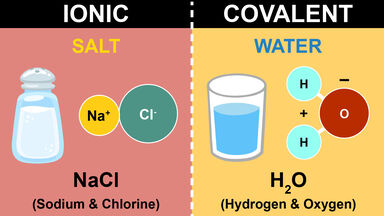
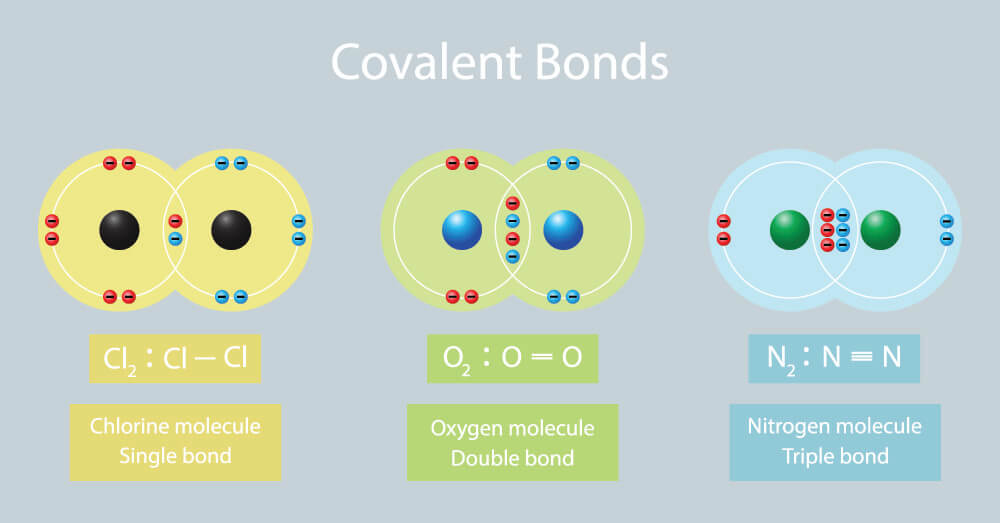
| Ionic Compounds | Covalent Compounds |
| Their constituent particles are ions and they are hard solids consisting of ions. This is because they have strong intermolecular forces of attraction that cannot be separated easily. | Their constituent particles are molecules. These are gasses, liquids or soft solids. Some exceptions are Graphite and Diamond. This is because they have weak forces of attraction between the molecules. |
| These are non-volatile and have high melting and boiling points. This is due to strong forces of attraction between the ions, so a large amount of energy is required to break the forces between the oppositely charged ions. | These are volatile compounds with lower melting and boiling points. Since they have weak intermolecular forces between their constituent molecules, therefore less energy is required to overcome the energy between them. |
| Ionic compounds are water soluble but insoluble in organic solvents. This is because Water is a polar molecule, it decreases the intermolecular forces of attraction. Also, there is a rule in chemistry that says Like dissolves like. Whereas the organic solvents like Benzene and Toluene are nonpolar compounds. | These are insoluble in water but soluble in organic solvents like Benzene and Toluene. This is because the organic solvents are nonpolar and so are the covalent compounds and hence they get dissolved in the organic compounds. |
| They do not conduct electricity in solid state, but act as a good conductor in the molten state. Intermolecular forces of attraction between ions in the solid state are very strong whereas in the fused or the molten state, the forces become weaker due to which ions get mobile. | They do not conduct electricity either in the solid, or the molten state. This is due to the absence of free electrons or ions in the covalent molecules. |