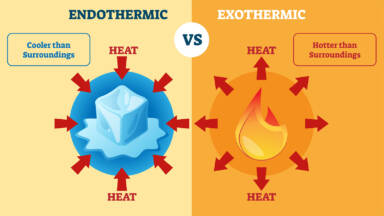
Question-What does one mean by exothermic and endothermic reactions? Give examples.
Answer-
Exothermic Reactions-
Exothermic reactions are the type of reaction in which heat is evolved or the reaction in which heat is released along with the formation of products are called exothermic reactions.
Examples of Exothermic Reactions-
Combustion of Natural Gas-
When Methane is burnt in the oxygen of air it forms carbon dioxide and water vapour. A large amount of heat is produced in this reaction.
CH4 (g)+ 2O2 (g)——CO2 (g)+ 2H20 (g)+ Heat Energy
The burning of methane gas is an exothermic reaction because heat is produced in this reaction.
Respiration-
During digestion Starch Carbohydrate is broken down into a simple carbohydrate glucose. This glucose then undergoes slow combustion by combining with oxygen in the cells of our body to produce energy in a process called respiration. In addition to other functions this energy maintains our body heat. During respiration, glucose combines with oxygen in the cells of our body to form carbon dioxide and water along with the production of energy.
C6H12O6 (aq) + 6O2 (g)———-6CO2 (g) + 6H2O (l) + Energy
Glucose + oxygen ——carbon dioxide + water + energy
Respiration is a type of exothermic process because energy is produced during this process.
Decomposition of Vegetable Matter into Compost-
This biological process releases heat as organic matter breaks down into simpler compounds.
Carbon burns in Oxygen to form Carbon Dioxide
When carbon burns in oxygen to form carbon dioxide, a lot of heat is produced in this reaction:
C (s)+O2 (g)—-CO2 (g)+HEAT
The burning of carbon in oxygen is an exothermic reaction because heat is evolved in this reaction.
Endothermic Reactions-
Endothermic reactions are those in which energy is absorbed from the surroundings in the form of heat, resulting in a decrease in temperature of the reaction mixture. These reactions require an input of energy to proceed.
Examples of Endothermic Reactions-
Decomposition of Calcium Carbonate-
All the decomposition reactions require energy to take place. So all the decomposition reactions are endothermic reactions
When calcium carbonate is heated it decomposes to form calcium oxide and carbon dioxide.
CaCO3 (s) + Heat——- CaO(s) + CO2
Calcium carbonate + Heat—— Calcium oxide + Carbon dioxide
The decomposition of calcium carbonate is an endothermic reaction because heat energy is absorbed in this reaction.
Thermal Decomposition of Ferrous Sulphate-
2FeSO₄(s) → Fe₂O₃(s) + SO₂(g) + SO₃(g)
Heating ferrous sulphate crystals leads to their decomposition into ferric oxide, sulphur dioxide, and sulphur trioxide, absorbing heat in the process.
Decomposition of Silver Chloride-
2AgCl(s) → 2Ag(s) + Cl₂(g)
Exposed to sunlight, silver chloride decomposes into silver and chlorine gas, an endothermic reaction utilising energy from light.
Photosynthesis
Photosynthesis is an endothermic reaction because sunlight energy is absorbed during the process of photosynthesis by the green plants.
Electrolysis
The electrolysis of water to form hydrogen and oxygen is also an endothermic reaction. This is because electric energy is absorbed during this reaction.
Examples of Endothermic reactions