Table of Contents
Introduction- What Are Chemical Elements?
A chemical element is a substance made up of only one kind of atom. It cannot be broken down into simpler substances using ordinary chemical methods. Each element is defined by the number of protons in the nucleus of its atoms-this number is called the atomic number. For example, all hydrogen atoms have exactly one proton and all oxygen atoms have eight.
Chemical elements are the basic building blocks of matter. Everything you see, touch, eat and breathe is made of chemical elements or combinations of them. Pure gold is made entirely of gold atoms (element symbol Au), while the oxygen we breathe is made of oxygen atoms bonded together in pairs (element symbol O₂).
To understand what chemical elements are is essential in science, especially in chemistry, biology, physics and Earth science. It helps explain how substances interact, why materials have specific properties and even how stars and planets form. For high school students, learning about elements provides a foundation to understand more advanced topics in science.
Chemical elements are found everywhere-not just in science labs or textbooks. In nature, elements make up rocks, oceans, the air and all living organisms. For instance-
- Carbon (C) is found in all living things.
- Iron (Fe) is in blood and the Earth’s core.
- Oxygen (O) makes up a large part of the air we breathe and the water we drink.
- Nitrogen (N) is also a major part of our atmosphere.
Beyond Earth, chemical elements are also present in stars and other celestial objects. The process of element formation in stars, called nucleosynthesis, produces new elements through nuclear fusion. This is how many of the heavier elements, such as calcium and iron, came into existence-eventually becoming part of the planets and living beings.
Currently, scientists recognize 118 chemical elements, each with its own unique name, symbol and properties. These elements are organized in the periodic table, a tool that shows how elements are related to each other based on their atomic structure and chemical behavior.
The Basics- Atoms and Elements
To understand chemical elements, it’s important to understand atoms, because all elements are made of atoms.
An atom is the smallest unit of matter that still retains the properties of an element. While extremely small, atoms are made up of even smaller particles-
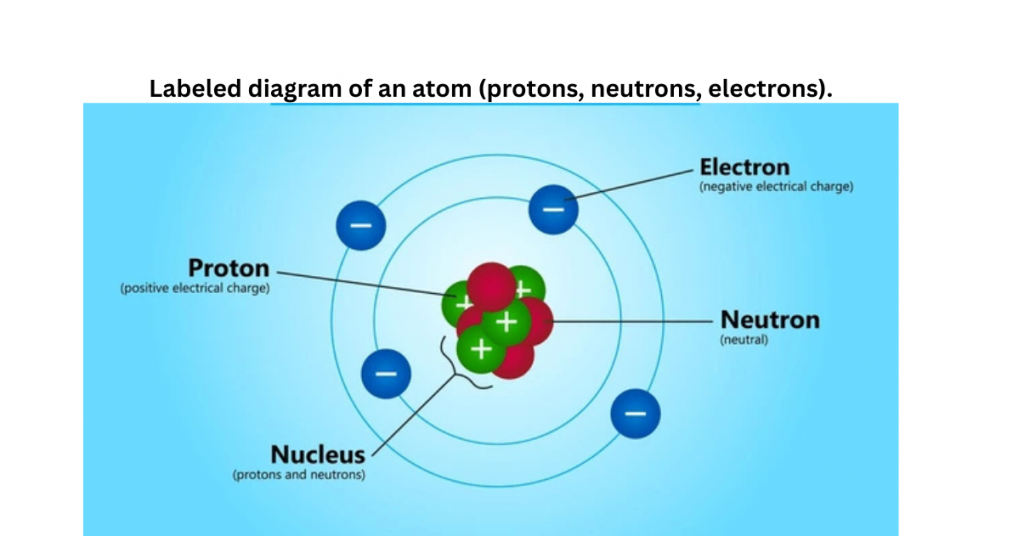
- Protons– positively charged particles found in the atom’s central core, or nucleus.
- Neutrons– neutral particles (no charge) also found in the nucleus.
- Electrons– negatively charged particles that move around the nucleus in regions called electron clouds or orbitals.
The atomic number of an element is the number of protons in its atoms. This number determines what element it is. For example-
- Hydrogen has 1 proton → atomic number 1.
- Helium has 2 protons → atomic number 2.
- Carbon has 6 protons → atomic number 6.
Every element’s atoms have the same number of protons, but the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. These isotopes still behave the same in most chemical reactions but may have different masses or stability. For example, carbon-12 and carbon-14 are isotopes of carbon; carbon-14 is radioactive and used in dating fossils.
The atomic mass (or mass number) of an atom is the total number of protons and neutrons in its nucleus. Since electrons have very little mass compared to protons and neutrons, they do not significantly affect atomic mass.
The structure and number of these subatomic particles determine the element’s chemical behavior. For example, how many electrons an atom has in its outer shell influences how it will bond with other atoms to form compounds.
Elements vs. Compounds vs. Mixtures

While elements are the simplest forms of matter, most substances in the world are not made of just one element. They are either compounds or mixtures. To understand how substances differ, we need to look at how atoms and elements interact.
Chemical Elements
A chemical element contains only one kind of atom. Elements are pure substances and have specific physical and chemical properties. Examples include-
- Hydrogen (H) – a gas that is highly flammable.
- Oxygen (O) – essential for respiration.
- Iron (Fe) – a metal used in construction and found in blood.
You cannot chemically break an element into anything simpler. Even if you apply heat or pass electricity through it, it stays the same substance.
Also Check – What Are Elements, Compounds, and Mixtures? Key Differences and Examples
Chemical Compounds
A compound forms when two or more different elements combine in a fixed ratio through a chemical reaction. The atoms in a compound are bonded together and the compound has entirely new properties compared to the elements it is made from.
Examples of compounds include-
- Water (H₂O)– made of hydrogen and oxygen. Hydrogen is flammable and oxygen supports burning, but together they form water, a liquid essential for life.
- Sodium chloride (NaCl)– made of sodium, a highly reactive metal and chlorine, a poisonous gas. Together, they form table salt, which is safe to eat.
Compounds can be broken down into elements only by chemical reactions, such as electrolysis (for water) or high-heat decomposition.
Mixtures
A mixture is a physical combination of two or more substances. In mixtures, elements or compounds are simply mixed together, not chemically bonded. Each substance in a mixture keeps its original properties and can usually be separated by physical means like filtering, evaporation, or using magnets.
Examples include-
- Air– a mixture of nitrogen, oxygen, carbon dioxide and other gases.
- Seawater– water mixed with salts and minerals.
- Steel– a mixture (alloy) of iron with small amounts of carbon and sometimes other metals.
Mixtures can be homogeneous (uniform throughout, like salt water) or heterogeneous (different parts visible, like a salad).
The main differences among elements, compounds and mixtures are-
- Elements consist of one kind of atom.
- Compounds consist of two or more elements bonded chemically.
- Mixtures are combinations of substances without chemical bonds.
The Periodic Table- Organizing Elements
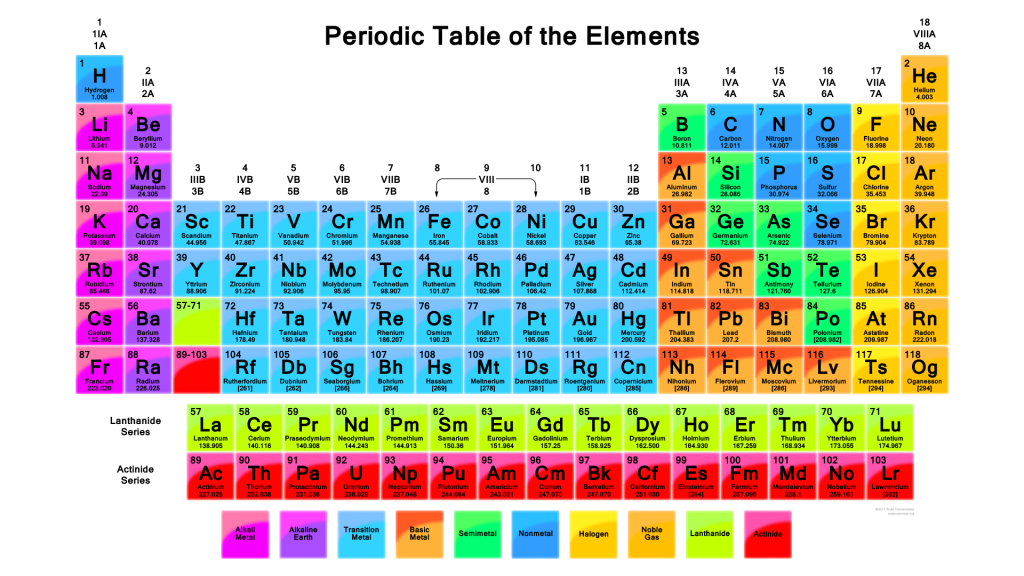
The periodic table is a scientific tool that organizes all known chemical elements based on their atomic number and chemical properties. It allows scientists and students to easily compare and understand the behavior of different elements.
The table was first developed by Dmitri Mendeleev in 1869. He arranged the elements in rows and columns based on their increasing atomic mass and noticed that certain properties repeated at regular intervals. Today’s version of the table is arranged by atomic number (the number of protons in an atom), which provides an even clearer structure.
Structure of the Periodic Table-
- Horizontal rows are called periods. There are 7 periods. As you move from left to right across a period, the atomic number increases and elements gradually change from metals to nonmetals.
- Vertical columns are called groups or families. Elements in the same group have similar chemical properties because they have the same number of electrons in their outer shells. For example, Group 1 elements (like sodium and potassium) are highly reactive metals known as alkali metals, while Group 18 contains the noble gases, which are very stable and unreactive.
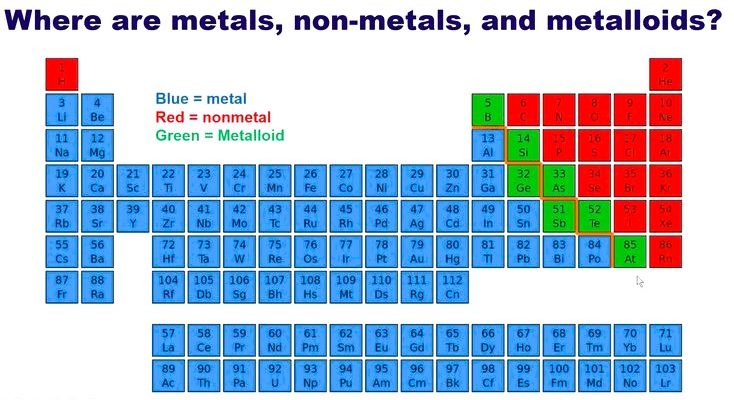
The table also separates elements into broad categories-
- Metals– good conductors of heat and electricity; usually solid and shiny.
- Nonmetals– poor conductors; can be gases or brittle solids.
- Metalloids– elements with properties of both metals and nonmetals.
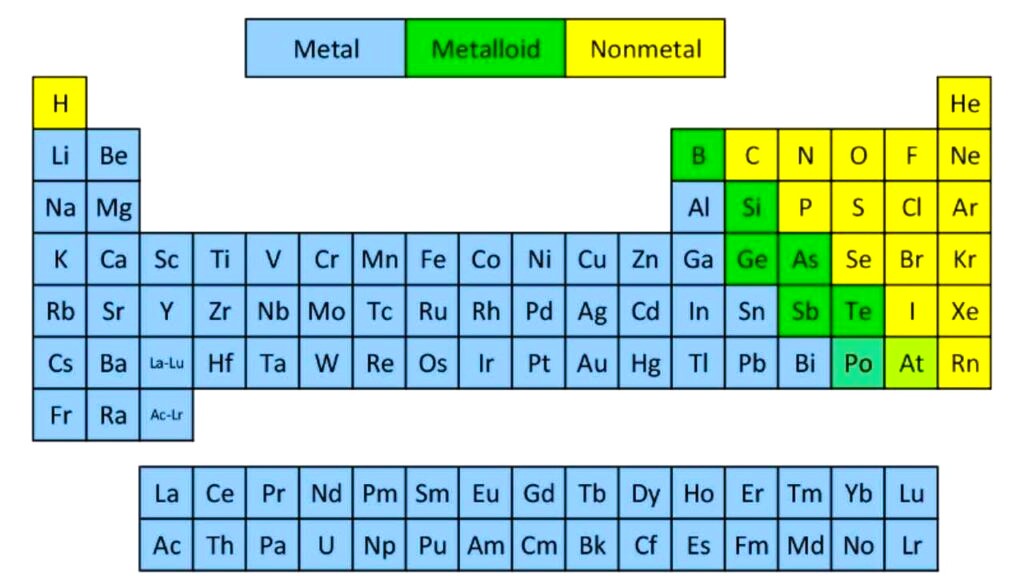
The lanthanides and actinides, which are placed in two separate rows at the bottom of the table, include elements used in electronics, nuclear power and medicine.
The periodic table is more than just a list-it reveals patterns. For instance, elements in the same group form similar compounds and react in comparable ways. This is because their electron arrangements determine how they bond with other elements.
Using the periodic table, students can predict how an element will behave in a reaction, identify its state at room temperature and understand its role in the natural and industrial world.
Also Check- The Periodic Table of Elements: What It Is and How It Works
Types and States of Elements
Chemical elements can be grouped not only by their position in the periodic table but also by their physical states and chemical types.
States of Matter at Room Temperature-
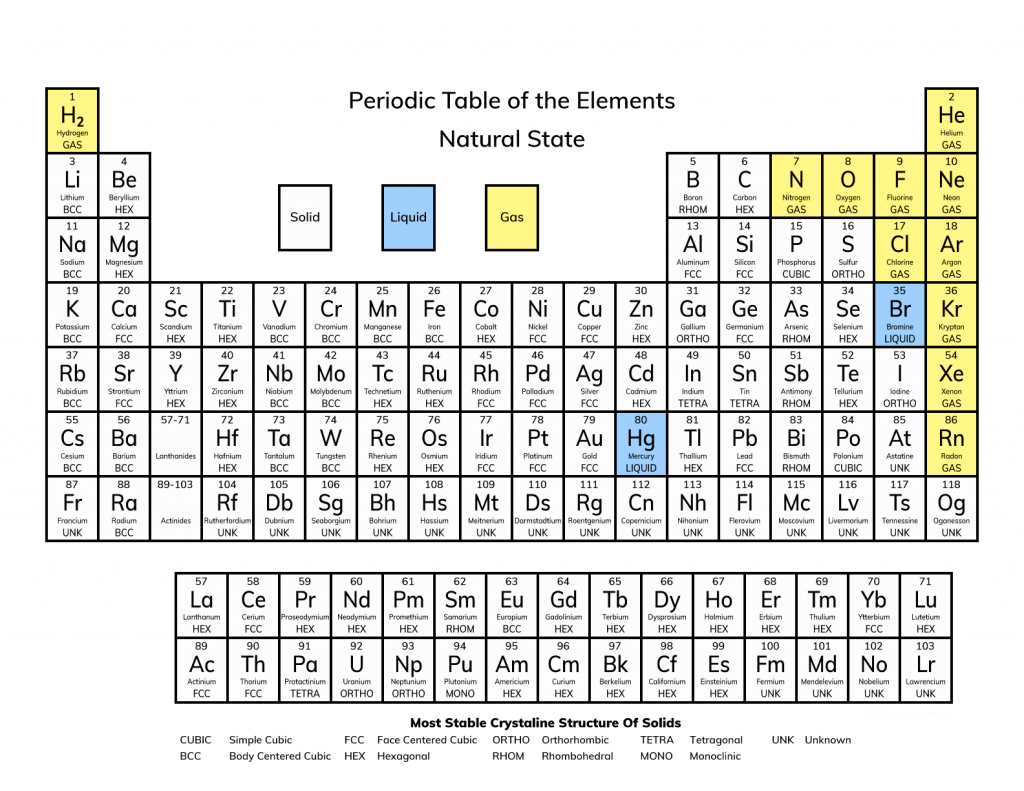
Out of the 118 known elements-
- 11 are gases– including hydrogen, nitrogen, oxygen and all six noble gases (like helium and neon).
- 2 are liquids– bromine (Br) and mercury (Hg).
- The rest are solids, including metals like iron, aluminum and gold.
A few elements, such as cesium and gallium, melt just above room temperature and may appear as soft solids or liquids depending on conditions.
Types of Elements-
- Metals
- Typically solid (except mercury).
- Shiny, malleable and good conductors of electricity and heat.
- Found on the left and center of the periodic table.
- Examples- copper, iron, zinc.
- Typically solid (except mercury).
- Nonmetals
- Found on the right side of the periodic table.
- Often gases or brittle solids.
- Poor conductors of heat and electricity.
- Examples- oxygen, carbon, sulfur.
- Found on the right side of the periodic table.
- Metalloids
- Elements that have properties of both metals and nonmetals.
- Often semiconductors, useful in electronics.
- Examples- silicon, arsenic, boron.
- Elements that have properties of both metals and nonmetals.
- Noble Gases
- Group 18 elements.
- Colorless, odorless and very stable.
- Do not easily form compounds.
- Examples- helium, neon, argon.
- Group 18 elements.
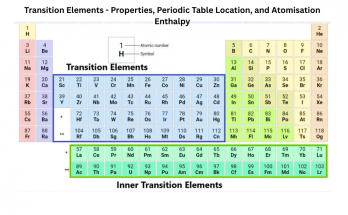
- Transition Metals
- Located in the central block (Groups 3 to 12).
- Hard, shiny and have high melting points.
- Often used in construction, electronics and alloys.
- Examples- gold, silver, nickel, titanium.
- Located in the central block (Groups 3 to 12).
Where Do Elements Come From? (Origin of Elements in the Universe)
Elements did not always exist. They were formed over billions of years through cosmic processes. The origin of elements tells the story of how matter evolved from simple particles into the complex materials that make up stars, planets and life itself.
The Big Bang and Light Elements-
According to the Big Bang theory, the universe began about 13.8 billion years ago. In the first few minutes, conditions were extremely hot and dense. During this time, hydrogen and helium formed through a process known as primordial nucleosynthesis. These two elements still make up over 98% of all visible matter in the universe.
Stellar Nucleosynthesis-
As the universe cooled and stars began to form, nuclear fusion inside stars created heavier elements. In the cores of stars, hydrogen atoms fuse into helium. As stars age and their core temperatures rise, they start fusing helium into elements like carbon, oxygen and neon.
In very massive stars, fusion continues all the way up to iron. Fusion reactions that produce elements heavier than iron require more energy than they release.
Supernovae and Heavy Elements-
When large stars exhaust their fuel, they may explode in massive events called supernovae. These explosions create enough energy to form the heaviest elements, including gold, uranium and lead, through a process known as rapid neutron capture, or the r-process.
These elements are then scattered into space, mixing with gas and dust. Eventually, they become part of new stars, planets and living organisms. In this way, many of the atoms in your body were formed in ancient stars-a concept often summarized by the phrase- we are made of stardust.
Formation of the Solar System-
When our solar system formed about 4.6 billion years ago, it inherited these elements from previous generations of stars. The Earth, Moon and meteorites all share similar chemical compositions, suggesting they came from the same pool of cosmic material.
Elements in Everyday Life
Chemical elements are not just theoretical ideas from textbooks-they are part of everything around us. From the air we breathe to the phones in our pockets, chemical elements are present in every part of our daily lives.
In the Human Body-
The human body is primarily made of a few key elements-
- Oxygen (O) – found in water and involved in breathing and energy production.
- Carbon (C) – the backbone of organic molecules like proteins and DNA.
- Hydrogen (H) – part of water and many biological compounds.
- Nitrogen (N) – a major component of proteins and nucleic acids.
- Calcium (Ca) – essential for bones and muscle function.
- Iron (Fe) – part of hemoglobin, which carries oxygen in the blood.
These elements make up about 99% of the atoms in your body.
In the Environment-
- Air is a mixture of elements like nitrogen, oxygen and argon.
- Water is a compound of hydrogen and oxygen but depends on the availability of both elements in nature.
- Soil contains minerals made of elements like phosphorus, potassium and magnesium, which plants need to grow.
In Technology and Industry-
Many everyday objects are built from or powered by specific elements-
- Silicon (Si) is used in computer chips and solar panels.
- Copper (Cu) is used in electrical wiring.
- Aluminum (Al) is lightweight and used in cars and packaging.
- Lithium (Li) is found in rechargeable batteries.
Isotopes- Different Versions of the Same Element
While all atoms of a given element have the same number of protons, they can differ in the number of neutrons. These variations are called isotopes.
What Is an Isotope?
An isotope is an atom of the same element that has the same number of protons but a different number of neutrons. This difference changes the atomic mass but not the chemical properties.
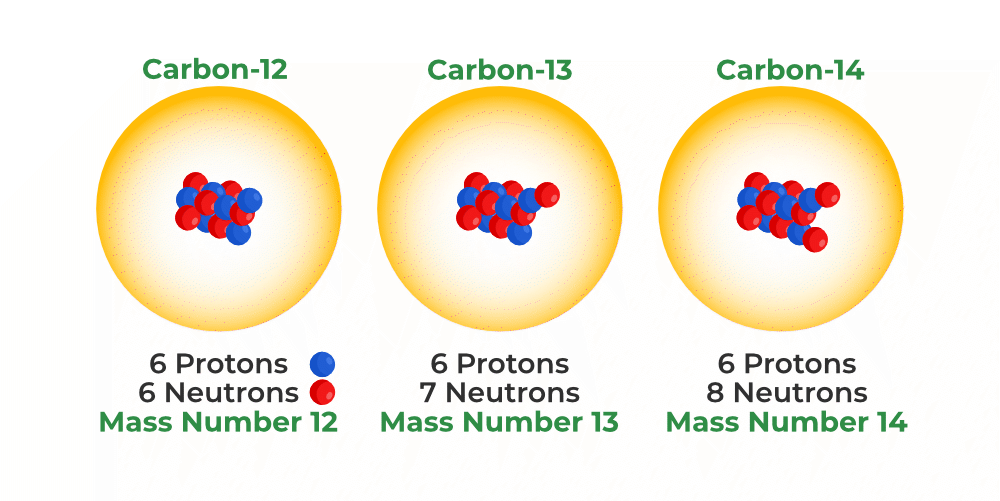
For example-
- Carbon-12 has 6 protons and 6 neutrons.
- Carbon-14 has 6 protons and 8 neutrons.
Both are carbon, but carbon-14 is radioactive and used in carbon dating to determine the age of ancient materials.
Stable vs. Radioactive Isotopes-
- Stable isotopes do not change over time.
- Radioactive isotopes decay into other elements and release radiation. This decay is used in-
- Medical imaging (e.g., iodine-131)
- Archaeology (carbon dating)
- Nuclear energy (uranium-235)
- Medical imaging (e.g., iodine-131)
Atomic Weight and Isotopes-
Since natural elements are mixtures of isotopes, their atomic weights are averages based on the relative abundance of each isotope. For example, the atomic weight of chlorine is about 35.45 because it is a mix of chlorine-35 and chlorine-37.
Knowing isotopes helps explain differences in atomic weights and why some forms of an element are useful for science and technology, while others may be unstable or even dangerous.
Fun Facts About Elements
Learning about chemical elements doesn’t have to be dry. Here are some fascinating facts that show how unique and surprising elements can be.
- Hydrogen is the most abundant element in the universe. It was also the first element formed after the Big Bang.
- Helium is used in balloons because it’s lighter than air and doesn’t burn.
- Mercury is the only metal that is liquid at room temperature.
- Gold and copper are the only metals that are naturally colored (others are silver or gray).
- Astatine is one of the rarest elements in the Earth’s crust-only a few grams exist naturally at any time.
- Oxygen makes up nearly half the mass of the Earth’s crust, mainly in the form of silicate minerals.
- Technetium was the first element made artificially and does not occur naturally in significant amounts.
Also Check – Nuclear Transmutation: How Elements Change in Nature and Science
Summary- Why Elements Matter
Chemical elements are the foundation of everything in the universe. From the air we breathe to the bones in our bodies, from the heat of the Sun to the structure of Earth’s crust, elements make up all matter-living and nonliving.
Each element has its own atomic structure that determines its chemical properties and together they form compounds and mixtures that drive every physical and biological process. Without an understanding of elements, we could not explain-
- How water forms from hydrogen and oxygen.
- Why carbon is central to life on Earth.
- How stars create heavier elements through nuclear fusion.
- Why certain materials conduct electricity while others do not.
Frequently Asked Questions
What is a chemical element in simple terms?
A chemical element is a pure substance made of only one kind of atom. It cannot be broken down into simpler substances by chemical means.
How many chemical elements are there?
There are 118 known chemical elements, with 94 found naturally and the rest made artificially in laboratories.
What’s the difference between an atom and an element?
An atom is the smallest particle of an element. An element is a substance made entirely of atoms that have the same number of protons.
What is the periodic table used for?
The periodic table organizes elements by their atomic number and chemical properties. It helps scientists predict how elements will react and interact.
Can elements be created or destroyed?
Elements cannot be destroyed by ordinary chemical reactions. They can change through nuclear reactions, such as those in stars or atomic reactors.
What are some examples of chemical elements in everyday life?
Oxygen (in air), iron (in blood and tools), calcium (in bones), carbon (in all living things) and silicon (in electronics) are all common elements.
Where did the elements come from?
Most elements were formed inside stars through nuclear fusion. Heavier elements formed during supernova explosions and were later incorporated into planets and life.
What are isotopes?
Isotopes are atoms of the same element with different numbers of neutrons. Some are stable, while others are radioactive and decay over time.
Glossary of Key Terms
Atom – The smallest unit of an element that retains its properties; made of protons, neutrons and electrons.
Atomic Number – The number of protons in the nucleus of an atom; determines which element it is.
Atomic Mass – The total number of protons and neutrons in an atom.
Chemical Element – A pure substance made of atoms with the same number of protons.
Compound – A substance formed when two or more elements chemically bond together.
Mixture – A combination of two or more substances that are not chemically bonded.
Isotope – Atoms of the same element with different numbers of neutrons.
Periodic Table – A chart that organizes chemical elements by atomic number and chemical properties.
Nucleosynthesis – The process by which new elements are formed inside stars.
Supernova – A powerful stellar explosion that creates and distributes heavy elements across space.
Metals – Elements that are usually shiny, conductive and malleable.
Nonmetals – Elements that are generally poor conductors and not shiny or malleable.
Metalloids – Elements that have properties of both metals and nonmetals.
Noble Gases – A group of elements (Group 18) that are inert and rarely react with other elements.