Table of Contents
Chapter 11 – Chemical Effects of Electric Current (Class 8)
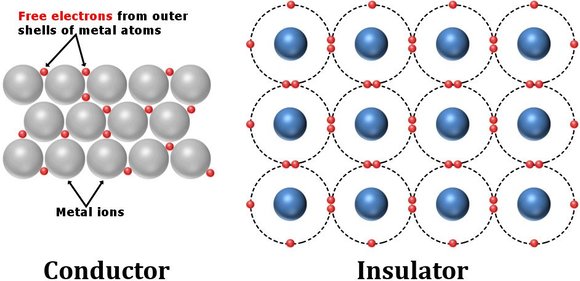
What Are Conductors?
Definition:
Conductors are materials that allow electric current to pass through them easily. They have free electrons in their atomic structure that move freely when a voltage is applied, enabling the flow of electricity.
Atomic Structure of Conductors
- Every atom consists of a nucleus (containing protons and neutrons) and electrons moving in orbits around the nucleus.
- In metals, the outermost (valence) electrons are loosely bound and can easily detach from their atoms.
- These free electrons move throughout the material, allowing electric current to pass through.
Key Features of Conductors
- Low Electrical Resistance: Conductors have low resistance, meaning electrons can move through them with minimal opposition.
- Free Electron Movement: Electrons are not tightly held to their atoms, allowing them to flow when a potential difference (voltage) is applied.
- Easy Flow of Electric Current: When a battery or voltage source is connected, the free electrons move in a specific direction, creating an electric current.
Examples of Conductors
1. Solid Conductors (Metals):
| Material | Why It Is a Good Conductor | Uses |
| Copper (Cu) | Low resistance, high conductivity | Electrical wires, cables |
| Aluminum (Al) | Lightweight, cost-effective | Overhead power lines |
| Gold (Au) | Corrosion-resistant, excellent conductivity | High-end electronics, circuit boards |
| Silver (Ag) | Best electrical conductor but expensive | Electrical contacts, satellites |
| Iron (Fe) | Conducts well but prone to rust | Electrical tools, transformers |
2. Liquid Conductors:
| Material | Why It Is a Conductor | Examples |
| Saltwater (NaCl solution) | Contains free-moving ions | Ocean water, electrolyte solutions |
| Acidic Solutions | Release charged ions | Lemon juice, vinegar, hydrochloric acid |
| Basic Solutions | Contain free-moving charged particles | Caustic soda, ammonia |
3. Gaseous Conductors:
| Material | Why It Is a Conductor | Examples |
| Ionized Air | High voltage ionizes air molecules | Lightning, neon signs |
| Plasma | Contains free-moving charged particles | Sun, fluorescent lights |
Real-Life Applications of Conductors
- Electrical Wiring: Copper and aluminum wires transmit electricity in homes, industries, and power plants.
- Electronics & Circuits: Gold and silver conductors are used in computer chips, mobile phones, and semiconductors.
- Batteries & Electrolytes: Conducting liquids like acids and salts are used in batteries to transfer charges.
- Electric Vehicles: Efficient transmission of electricity within the vehicle’s motor and battery system.
- Lightning Rods: Copper rods safely conduct lightning into the ground.
Also Check – 15 Shockingly Cool Facts About Conductors & Insulators!
What Are Insulators?
Definition:
Insulators are materials that do not allow electric current to pass through them easily. Their electrons are tightly bound to their atoms, which restricts the movement of electric charge.
Atomic Structure of Insulators
- In insulators, electrons are strongly held by their atoms.
- There are no free electrons available to move and carry electric current.
- This prevents the flow of electricity through the material.
Key Features of Insulators
- High Electrical Resistance: Insulators oppose the flow of electric current.
- Tightly Bound Electrons: Electrons are held tightly to the nucleus, preventing free movement.
- Prevention of Current Flow: This makes them useful for electrical insulation and safety.
Examples of Insulators
1. Solid Insulators:
| Material | Why It Is an Insulator | Uses |
| Rubber | High resistance, flexible | Wire insulation, gloves |
| Plastic | Lightweight, durable | Electric sockets, switches |
| Wood | Non-conductive unless wet | Tool handles, furniture |
| Glass | Very high resistance | High-voltage power line insulators |
| Ceramics | Heat-resistant and non-conductive | Electrical insulators in power stations |
2. Liquid Insulators:
| Material | Why It Is an Insulator | Examples |
| Distilled Water | No free-moving ions | Used in chemistry experiments |
| Oils (e.g., transformer oil) | Prevents electrical discharge | Used in transformers, cooling |
| Alcohol | Does not contain charged particles | Used in some electrical devices |
3. Gaseous Insulators:
| Material | Why It Is an Insulator | Examples |
| Air (at normal conditions) | Does not allow current flow | Used in circuit breakers |
| Sulfur Hexafluoride (SF₆) | Strong insulating gas | Used in high-voltage systems |
Real-Life Applications of Insulators
- Electrical Safety: Plastic and rubber insulate electrical wires to prevent electric shocks.
- High Voltage Power Lines: Glass and ceramic insulators prevent electricity from escaping transmission lines.
- Transformer Cooling: Special insulating oils are used to prevent overheating.
- Lightning Protection: Buildings are insulated to prevent electric shocks from lightning.
- Heat Insulation: Wood, rubber, and ceramic prevent heat loss in appliances like ovens and refrigerators.
Also Check – The Science Behind Conductors and Insulators – A Step-by-Step Explanation
Difference between Conductor and Insulator
| Feature | Conductors | Insulators |
| Definition | Allow electric current to flow easily | Do not allow electric current to flow |
| Electron Movement | Free electrons move easily | Electrons are tightly bound |
| Electrical Resistance | Low | High |
| Examples | Copper, Aluminum, Silver | Rubber, Plastic, Wood |
| Applications | Wiring, batteries, circuits | Wire coatings, safety equipment |
Conductivity of Liquids
Electricity does not only flow through solid conductors like metals—it can also pass through liquids under certain conditions. The conductivity of liquids is essential for applications such as batteries, electrolysis, and water purification.
Testing the Conductivity of Liquids Using a Circuit with a Bulb
Materials Needed
- A battery (power source)
- A small bulb (or LED)
- Connecting wires
- Two electrodes (e.g., carbon rods, metal plates, or nails)
- Different liquids to test (e.g., distilled water, saltwater, vinegar, oil, etc.)
Steps to Test Conductivity
- Set Up the Circuit:
- Connect the positive terminal of the battery to one electrode.
- Attach a wire from the other electrode to the bulb.
- Connect the bulb’s other end to the negative terminal of the battery.
- Test Different Liquids:
- Immerse both electrodes into the liquid to be tested.
- Observe whether the bulb glows.
- Interpret the Results:
- If the bulb glows, the liquid conducts electricity.
- If the bulb does not glow, the liquid does not conduct electricity.
Observations Table
| Liquid Tested | Bulb Glows? | Conclusion |
| Saltwater | Yes | Good Conductor |
| Vinegar (Acid) | Yes | Good Conductor |
| Tap Water | Dim Glow | Weak Conductor |
| Distilled Water | No | Poor Conductor |
| Oil | No | Insulator |
| Sugar Solution | No | Poor Conductor |
Why Some Liquids Conduct Electricity
Principle:
The ability of a liquid to conduct electricity depends on the presence of free-moving charged particles (ions).
Liquids That Conduct Electricity (Good Conductors)
- Electrolytes: Liquids containing ions (charged particles) such as:
- Acidic solutions (lemon juice, vinegar, hydrochloric acid)
- Basic solutions (sodium hydroxide, potassium hydroxide)
- Salt solutions (saltwater, copper sulfate solution)
- These liquids contain positive ions (cations) and negative ions (anions) that move freely and carry electric charge when a voltage is applied.
Liquids That Do Not Conduct Electricity (Poor Conductors)
- Non-electrolytes: Liquids that do not have free ions such as:
- Distilled water (pure H₂O)
- Oil (vegetable oil, petrol, diesel)
- Alcohol (ethanol, isopropyl alcohol)
- Sugar solutions (sugar dissolved in water)
- Without ions, no electric charge can be transferred, preventing current flow.
Factors Affecting the Conductivity of Liquids
- Presence of Dissolved Salts, Acids, or Bases:
These substances produce ions when dissolved, increasing conductivity. - Concentration of Ions:
Higher ion concentration leads to better conductivity. - Temperature:
Higher temperature allows ions to move faster, improving conductivity. - Type of Substance Dissolved:
Strong electrolytes (e.g., HCl, NaCl, H₂SO₄) fully dissociate into ions, while weak electrolytes (e.g., vinegar, ammonia) partially dissociate.
Use of LED in Place of a Bulb for Weak Current Detection
- LEDs glow with weak currents: They are more sensitive than bulbs, energy-efficient, and work well in low-voltage circuits.
- Testing: Replace the bulb with an LED; if it glows, the liquid conducts electricity—even if the current is weak.
Magnetic Effect of Electric Current for Conductivity Testing
Principle:
When a liquid conducts a very small current that may not light a bulb or LED, the magnetic effect can be used.
How It Works:
- Electric current produces a magnetic field.
- A compass needle placed near the circuit will deflect if current passes through the liquid.
Steps for Testing with a Compass:
- Set up the circuit as described above.
- Place a magnetic compass near the wire.
- Dip the electrodes into the liquid.
- Observation:
- If the needle moves, current is flowing (the liquid is a conductor).
- If the needle does not move, the liquid is an insulator.
Summary and Key Takeaways on Liquid Conductivity
- Some liquids conduct electricity while others do not.
- Liquids containing dissolved ions (acids, bases, salts) are good conductors.
- Distilled water, oil, and alcohol do not conduct electricity because they lack free-moving ions.
- The conductivity of a liquid depends on the type and concentration of dissolved substances.
- An LED and a magnetic compass are effective tools for detecting weak electric currents.
Real-Life Applications of Liquid Conductivity
- Water Purification: Conductivity tests help determine water quality and purity.
- Battery Electrolytes: Car batteries use sulfuric acid to conduct electricity.
- Electrolysis: Used for metal plating, hydrogen production, and chemical industries.
- Medical Uses: Electrolyte balance is tested in blood samples for health monitoring.
Effects of Electric Current
Electric current produces several effects when it flows through different materials. The three main effects are:
- Heating Effect of Electric Current
- Magnetic Effect of Electric Current
- Chemical Effects of Electric Current
1. Heating Effect of Electric Current (Brief Overview)
- Process: Occurs when electrical energy is converted into heat energy.
- Governing Law: Joule’s Law, expressed as:
H = I² R t
where H is heat produced, I is current, R is resistance, and t is time. - Examples: Electric heaters, irons, toasters, ovens, fuses, and electric bulbs.
2. Magnetic Effect of Electric Current
- Process: When an electric current passes through a conductor, it produces a magnetic field.
- Application in Conductivity Testing:
If a liquid conducts only a weak current, a magnetic compass placed near the circuit can detect the magnetic field. - Experiment Steps:
- Set up the circuit with a battery, wires, and electrodes.
- Place a magnetic compass near the circuit.
- Immerse the electrodes in the liquid.
- Observe the compass needle for deflection.
- Applications: Electric motors, electromagnets, magnetic relays, and circuit breakers.
3. Chemical Effects of Electric Current
- Process: When electric current passes through a conducting liquid, it can cause chemical changes.
- Effects:
- Formation of gas bubbles at the electrodes.
- Deposition of metal on electrodes.
- Change in color of the solution.
- Formation of new substances in the liquid.
- Examples: Electrolysis of water, electroplating, and metal purification.
Electrolysis of Water
- Process:
When an electric current is passed through water (with a small amount of acid or salt), it splits into hydrogen (H₂) and oxygen (O₂) gas. - Experiment Steps:
- Fill a beaker with water and add a few drops of acid.
- Immerse two electrodes in the water.
- Cover each electrode with an inverted test tube.
- Connect the electrodes to a battery.
- Gas bubbles appear at the electrodes.
- Observations:
- Cathode produces hydrogen gas.
- Anode produces oxygen gas.
- The volume of hydrogen is approximately twice that of oxygen.
- Chemical Reaction:
2H₂O → 2H₂ + O₂
Color Changes Due to Electric Current
- Example:
In a copper sulfate solution with copper electrodes, the cathode gets coated with copper, causing the solution to fade in color.
Real-Life Applications of the Chemical Effects of Electric Current
- Electroplating: Coating one metal with another (e.g., gold-plated jewelry, chrome-plated bike parts).
- Metal Refining: Purifying impure metals such as copper and aluminum.
- Battery Operation: Chemical reactions inside batteries generate electricity.
- Water Purification: Electrolysis is used to remove impurities from water.
- Medical Applications: Electrolysis in hair removal treatments and manufacturing medicines.
Summary and Key Takeaways on Electric Current Effects
- The three effects of electric current are heating, magnetic, and chemical.
- The magnetic effect can be used to detect weak currents.
- The chemical effect leads to reactions such as gas formation, metal deposition, and color changes.
- Electrolysis is a process where electricity breaks down a liquid into simpler substances.
Testing Various Liquids for Conductivity
Electricity can pass through some liquids but not all. The ability of a liquid to conduct electricity depends on the presence of ions (charged particles).
Conductivity Testing Setup
Materials Needed
- Battery (power source)
- Bulb (or LED)
- Connecting wires
- Two electrodes (metal strips, carbon rods, or iron nails)
- Liquids to test (tap water, distilled water, lemon juice, vinegar, saltwater, etc.)
Testing Steps
- Set Up the Circuit:
- Connect one electrode to the positive terminal of the battery.
- Connect the other electrode to one terminal of the bulb.
- Attach a wire from the bulb to the negative terminal of the battery.
- Test Different Liquids:
- Immerse the electrodes in the liquid sample.
- Observe whether the bulb glows.
- Observations:
- If the bulb glows, the liquid is a good conductor.
- If the bulb does not glow, the liquid is a poor conductor.
Tap Water vs. Distilled Water
Tap Water:
- Conducts electricity due to dissolved salts and minerals (e.g., sodium, calcium, magnesium, chloride).
Conclusion: Good conductor.
Distilled Water:
- Does not conduct electricity because it is pure H₂O with no dissolved salts.
Conclusion: Poor conductor.
Experiment Results
| Type of Water | Bulb Glows? | Conclusion |
| Tap Water | Yes | Good Conductor |
| Distilled Water | No | Poor Conductor |
Conductivity of Acid, Base, and Salt Solutions
- Acidic Solutions:
Release hydrogen ions (H⁺) in water.
Examples: Lemon juice, vinegar, hydrochloric acid, sulfuric acid. - Basic Solutions:
Release hydroxide ions (OH⁻) in water.
Examples: Sodium hydroxide, potassium hydroxide, ammonia solution. - Salt Solutions:
Dissolve to form positive and negative ions.
Examples: Table salt solution, copper sulfate solution, potassium chloride solution.
Experiment Results
| Solution | Bulb Glows? | Conclusion |
| Lemon Juice (Citric Acid) | Yes | Good Conductor |
| Vinegar (Acetic Acid) | Yes | Good Conductor |
| Sodium Hydroxide (NaOH) | Yes | Good Conductor |
| Salt Solution (NaCl) | Yes | Good Conductor |
Conductivity of Fruit and Vegetable Extracts
Some fruits and vegetables naturally contain acids, salts, and minerals that make them conductive.
| Fruit/Vegetable Extract | Bulb Glows? | Conclusion |
| Lemon Juice | Yes | Good Conductor |
| Vinegar | Yes | Good Conductor |
| Potato Juice | Dim Glow | Weak Conductor |
| Tomato Juice | Dim Glow | Weak Conductor |
Role of Impurities in Making Water Conductive
- Pure Water (Distilled): Does not conduct electricity.
- Water with Impurities:
Adding substances like salt, acids, or minerals introduces ions, making water conductive.
Example: Testing distilled water first (no bulb glow), then adding a pinch of salt causes the bulb to glow.
Summary and Key Takeaways on Liquid Conductivity
- Tap water conducts electricity due to dissolved salts, while distilled water does not.
- Acids, bases, and salts conduct electricity by releasing free ions.
- Fruit and vegetable extracts can be conductive depending on their natural content.
- Impurities such as salts and acids make water conductive.
Electroplating – Detailed Explanation
Electroplating is the process of depositing a thin layer of metal onto another metal object using electric current. It improves appearance, prevents corrosion, and increases durability.
Process of Electroplating
Materials Required
- A battery or power source
- Two electrodes (metal plates or rods)
- An electrolyte solution (a salt solution of the metal to be plated)
- The object to be electroplated
Steps in the Electroplating Process
- Set Up the Circuit:
- Connect the positive terminal of the battery to the metal that will supply the coating (anode).
- Connect the negative terminal to the object to be electroplated (cathode).
- Prepare the Electrolyte Solution:
- The electrolyte should be a solution of the metal to be deposited (e.g., copper sulfate for copper plating).
- Immerse the Electrodes:
- Place both the anode and the object (cathode) in the electrolyte.
- Pass Electric Current:
- Metal ions move from the anode towards the cathode and deposit on the object.
- Observation:
- A thin layer of metal gradually coats the object.
Example: Copper Electroplating Reaction
- At the Anode (Positive Electrode):
Copper atoms dissolve into the solution:
Cu → Cu²⁺ + 2e⁻ - At the Cathode (Negative Electrode):
Copper ions deposit onto the object:
Cu²⁺ + 2e⁻ → Cu
Purpose and Applications of Electroplating
- Protection Against Corrosion:
Prevents rusting by forming a protective metal layer.
Example: Galvanization (zinc plating on iron). - Improving Appearance:
Provides a shiny, attractive finish.
Example: Gold or silver plating on jewelry. - Increasing Durability:
Electroplated surfaces resist wear and tear.
Example: Chrome-plated car parts. - Cost Efficiency:
Makes cheaper metals look expensive by adding a thin layer of valuable metal.
Real-World Examples
- Automobile Industry: Chrome-plated car parts (bumpers, mirrors) to prevent rust.
- Food Industry: Tin-plated food cans prevent contamination and rust.
- Jewelry: Gold or silver plating on inexpensive metals.
- Electronics: Copper electroplating to enhance circuit conductivity.
- Aerospace and Marine: Nickel and chrome electroplating for corrosion protection.
Environmental Concerns in Electroplating
- Toxic Wastewater: Contains toxic metals like chromium and nickel.
- Air Pollution: Release of toxic gases can affect air quality.
- Soil Contamination: Heavy metals in waste can pollute soil.
- Health Hazards: Workers may be exposed to toxic chemicals.
Reducing Environmental Impact
- Treatment of wastewater before disposal.
- Recycling of chemicals.
- Using non-toxic electroplating solutions.
- Adhering to strict government regulations.
Summary and Key Takeaways on Electroplating
- Electroplating deposits a thin metal layer using electric current.
- It is used for protection, decoration, and enhancing durability.
- Commonly electroplated objects include car parts, food cans, and jewelry.
- Environmental concerns require proper waste management and recycling.
Applications and Real-World Implications of Electricity
Electricity plays a crucial role in daily life but comes with risks that require proper safety measures.
1. Safety When Using Electrical Appliances with Wet Hands
- Why Wet Hands Are Dangerous:
Water with dissolved salts conducts electricity. Wet skin increases the risk of electric shock. - Safety Measures:
- Dry your hands before using electrical devices.
- Avoid using electrical appliances near water.
- Ensure outlets in wet areas are properly grounded.
2. Firefighters and Electrical Safety
- Why Firefighters Turn Off Electricity:
Water is a good conductor and can lead to electrocution if used on live electrical wires. - Safety Measures for Firefighters:
- Turn off the main power supply before using water hoses.
- Use specialized fire extinguishers for electrical fires.
- Wear insulated gear such as rubber gloves and boots.
3. Seawater vs. Freshwater Conductivity
- Seawater:
High conductivity due to dissolved salts (e.g., sodium chloride). - Freshwater:
Low conductivity because it has fewer dissolved salts. - Real-World Implications:
- Proper grounding for boats and ships.
- Use in ocean-based power generation and climate studies.
4. Corrosion Prevention Through Electroplating
- Corrosion Process:
Metals react with oxygen and water, leading to rust. - Electroplating as Prevention:
Coats metal with a corrosion-resistant layer (e.g., chromium, nickel, or zinc). - Applications:
Bridges, vehicles, and food cans.
5. General Electrical Safety Precautions
- Common Hazards:
Exposed wires, overloaded circuits, wet environments, and damaged appliances. - Safety Tips:
- Avoid touching electrical devices with wet hands.
- Always turn off power before repairs.
- Use insulated tools and proper protective equipment.
- Ensure proper grounding of appliances and circuits.
Summary and Key Takeaways on Electrical Safety
- Avoid using electrical devices with wet hands.
- Firefighters turn off electricity before using water hoses to prevent electrocution.
- Seawater is a better conductor than freshwater due to high salt content.
- Electroplating prevents corrosion by adding a protective metal layer.
- Always follow safety precautions when handling electricity to prevent shocks, fires, and accidents.